Plumbagin Enhances the Anticancer Effects of PF Chemotherapy via Downregulation of the PI3K/AKT/mTOR/p70S6K Pathway in Human Tongue Squamous Cell Carcinoma
- PMID: 36814557
- PMCID: PMC9940972
- DOI: 10.1155/2023/8306514
Plumbagin Enhances the Anticancer Effects of PF Chemotherapy via Downregulation of the PI3K/AKT/mTOR/p70S6K Pathway in Human Tongue Squamous Cell Carcinoma
Abstract
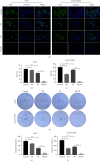
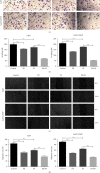
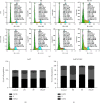
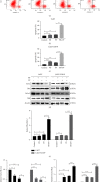
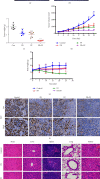
Cisplatin plus 5-fluorouracil (PF) is used as the standard neoadjuvant chemotherapy (also called preoperative chemotherapy) in the treatment of tongue squamous cell carcinoma (TSCC). Although PF chemotherapy reduces the distant metastasis of TSCC, the five-year survival rate has not significantly improved. In recent years, components considered in traditional Chinese medicine have been researched as adjuvant drugs for radiotherapy and chemotherapy. Plumbagin (PB) is a quinone component isolated from Plumbago zeylanica L. Notably, PB demonstrates numerous anticancer properties. In order to examine the chemosensitization effect of PB on PF and its associated mechanisms, in vitro experiments using TSCC Cal27 and cisplatin (CDDP)-resistant Cal27/CDDP cells were carried out in the present study, and the results were subsequently verified using nude mice xenografts. Results of the present study demonstrated that PB enhanced the anticancer effects of PF on the proliferation, migration, and invasion of Cal27 and Cal27/CDDP cells. Cell cycle assays demonstrated that both Cal27 and Cal27/CDDP cells were arrested in the S phase following the combined treatment of PF and PB. Moreover, the PF and PB combination group induced higher levels of apoptosis in Cal27 and Cal27/CDDP cells compared with the group treated with PF alone. In addition, the results of the present study demonstrated that combined PB and PF inhibited the PI3K/AKT/mTOR/p70S6K pathway in TSCC cells. Moreover, the weight and volumes of tumors in nude mice were reduced following treatment with a combination of PF and PB. Results of the present study also demonstrated that the expression levels of Ki67 were markedly reduced in the combined treatment group compared with the group treated with PF alone. In summary, the results of the present study demonstrated that PB enhanced the PF sensitivity of TSCC through induction of S-phase arrest and apoptosis via the PI3K/AKT/mTOR/p70S6K pathway.
Copyright © 2023 Shu-Ting Pan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
CXCR4 enhances cisplatin resistance of human tongue squamous cell carcinoma.J Oral Pathol Med. 2019 Feb;48(2):122-128. doi: 10.1111/jop.12813. Epub 2018 Dec 30. J Oral Pathol Med. 2019. PMID: 30554431
-
Plumbagin Enhances the Anticancer Efficacy of Cisplatin by Increasing Intracellular ROS in Human Tongue Squamous Cell Carcinoma.Oxid Med Cell Longev. 2020 Mar 25;2020:5649174. doi: 10.1155/2020/5649174. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32308804 Free PMC article.
-
Plumbagin has an inhibitory effect on the growth of TSCC PDX model and it enhances the anticancer efficacy of cisplatin.Aging (Albany NY). 2023 Nov 3;15(21):12225-12250. doi: 10.18632/aging.205175. Epub 2023 Nov 3. Aging (Albany NY). 2023. PMID: 37925175 Free PMC article.
-
Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells.Drug Des Devel Ther. 2015 Mar 16;9:1601-26. doi: 10.2147/DDDT.S76057. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834400 Free PMC article.
-
miR-22/KAT6B axis is a chemotherapeutic determiner via regulation of PI3k-Akt-NF-kB pathway in tongue squamous cell carcinoma.J Exp Clin Cancer Res. 2018 Jul 24;37(1):164. doi: 10.1186/s13046-018-0834-z. J Exp Clin Cancer Res. 2018. PMID: 30041677 Free PMC article.
Cited by
-
Biological Potential of Carnivorous Plants from Nepenthales.Molecules. 2023 Apr 21;28(8):3639. doi: 10.3390/molecules28083639. Molecules. 2023. PMID: 37110873 Free PMC article. Review.
-
Plumbagin targets the GLUT1/MMP-2 axis to inhibit oral squamous cell carcinoma progression.Cancer Cell Int. 2025 Jul 28;25(1):283. doi: 10.1186/s12935-025-03915-7. Cancer Cell Int. 2025. PMID: 40722096 Free PMC article.
-
Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways.J Cell Mol Med. 2024 Jul;28(13):e18386. doi: 10.1111/jcmm.18386. J Cell Mol Med. 2024. PMID: 38990057 Free PMC article.
-
Dysregulated PI3K/AKT signaling in oral squamous cell carcinoma: The tumor microenvironment and epigenetic modifiers as key drivers.Oncol Res. 2025 Jul 18;33(8):1835-1860. doi: 10.32604/or.2025.064010. eCollection 2025. Oncol Res. 2025. PMID: 40746882 Free PMC article. Review.
References
-
- Fang Q., Li P., Qi J. Value of lingual lymph node metastasis in patients with squamous cell carcinoma of the tongue. The Laryngoscope . 2019;129(11):2527–2530. - PubMed
-
- da Silva Souto A. C., Vieira Heimlich F., Lima de Oliveira L. Epidemiology of tongue squamous cell carcinoma: a retrospective cohort study. Oral Diseases . 2021;29(2) - PubMed
-
- Paterson C., Robertson A. G., Grose D. Neoadjuvant chemotherapy prior to surgery in head and neck cancer. Clinical Oncology . 2012;24(1):79–80. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

