Optogenetic control of beta-carotene bioproduction in yeast across multiple lab-scales
- PMID: 36814715
- PMCID: PMC9939774
- DOI: 10.3389/fbioe.2023.1085268
Optogenetic control of beta-carotene bioproduction in yeast across multiple lab-scales
Abstract
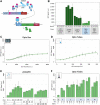
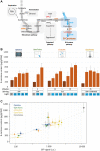
Optogenetics arises as a valuable tool to precisely control genetic circuits in microbial cell factories. Light control holds the promise of optimizing bioproduction methods and maximizing yields, but its implementation at different steps of the strain development process and at different culture scales remains challenging. In this study, we aim to control beta-carotene bioproduction using optogenetics in Saccharomyces cerevisiae and investigate how its performance translates across culture scales. We built four lab-scale illumination devices, each handling different culture volumes, and each having specific illumination characteristics and cultivating conditions. We evaluated optogenetic activation and beta-carotene production across devices and optimized them both independently. Then, we combined optogenetic induction and beta-carotene production to make a light-inducible beta-carotene producer strain. This was achieved by placing the transcription of the bifunctional lycopene cyclase/phytoene synthase CrtYB under the control of the pC120 optogenetic promoter regulated by the EL222-VP16 light-activated transcription factor, while other carotenogenic enzymes (CrtI, CrtE, tHMG) were expressed constitutively. We show that illumination, culture volume and shaking impact differently optogenetic activation and beta-carotene production across devices. This enabled us to determine the best culture conditions to maximize light-induced beta-carotene production in each of the devices. Our study exemplifies the stakes of scaling up optogenetics in devices of different lab scales and sheds light on the interplays and potential conflicts between optogenetic control and metabolic pathway efficiency. As a general principle, we propose that it is important to first optimize both components of the system independently, before combining them into optogenetic producing strains to avoid extensive troubleshooting. We anticipate that our results can help designing both strains and devices that could eventually lead to larger scale systems in an effort to bring optogenetics to the industrial scale.
Keywords: DIY; Optogenetics; Saccharomyces cerevisiae; beta-carotene; bioproduction; metabolic engineering; synthetic biology; yeast.
Copyright © 2023 Pouzet, Cruz-Ramón, Le Bec, Cordier, Banderas, Barral, Castaño-Cerezo, Lautier, Truan and Hersen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bogacz-Radomska L., Harasym J. (2018). β-Carotene-properties and production methods. Food Qual. Saf. 2 (2), 69–74. 10.1093/fqsafe/fyy004 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

