Preparing for the Next Influenza Season: Monitoring the Emergence and Spread of Antiviral Resistance
- PMID: 36814825
- PMCID: PMC9939793
- DOI: 10.2147/IDR.S389263
Preparing for the Next Influenza Season: Monitoring the Emergence and Spread of Antiviral Resistance
Abstract
Purpose: The relaxation of pandemic restrictions in 2022 has led to a reemergence of respiratory virus circulation worldwide and anticipation of substantial influenza waves for the 2022/2023 Northern Hemisphere winter. Therefore, the antiviral susceptibility profiles of human influenza viruses circulating in Germany were characterized.
Methods: Between October 2019 (week 40/2019) and March 2022 (week 12/2022), nasal swabs from untreated patients with acute respiratory symptoms were collected in the national German influenza surveillance system. A total of 598 influenza viruses were isolated and analyzed for susceptibility to oseltamivir, zanamivir and peramivir, using a neuraminidase (NA) inhibition assay. In addition, next-generation sequencing was applied to assess molecular markers of resistance to NA, cap-dependent endonuclease (PA) and M2 ion channel inhibitors (NAI, PAI, M2I) in 367 primary clinical samples. Furthermore, a genotyping assay based on RT-PCR and pyrosequencing to rapidly assess the molecular resistance marker PA-I38X in PA genes was designed and established.
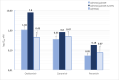
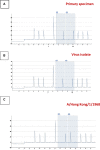
Results: While NAI resistance in the strict sense, defined by a ≥ 10-fold (influenza A) or ≥5-fold (influenza B) increase of NAI IC50, was not detected, a subtype A(H1N1)pdm09 isolate displayed 2.3- to 7.5-fold IC50 increase for all three NAI. This isolate carried the NA-S247N substitution, which is known to enhance NAI resistance induced by NA-H275Y. All sequenced influenza A viruses carried the M2-S31N substitution, which confers resistance to M2I. Of note, one A(H3N2) virus displayed the PA-I38M substitution, which is associated with reduced susceptibility to the PAI baloxavir marboxil. Pyrosequencing analysis confirmed these findings in the original clinical specimen and in cultured virus isolate, suggesting sufficient replicative fitness of this virus mutant.
Conclusion: Over the last three influenza seasons, the vast majority of influenza viruses in this national-level sentinel were susceptible to NAIs and PAIs. These findings support the use of antivirals in the upcoming influenza season.
Keywords: antiviral resistance; baloxavir marboxil; cap-dependent endonuclease; genotypic assay; influenza viruses; molecular resistance marker; neuraminidase; phenotypic assay; surveillance.
© 2023 Oh et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

