Rhodium-catalyzed selenylation and sulfenylation of quinoxalinones 'on water'
- PMID: 36814880
- PMCID: PMC9940630
- DOI: 10.1039/d2ra07400a
Rhodium-catalyzed selenylation and sulfenylation of quinoxalinones 'on water'
Abstract
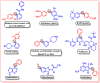
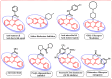
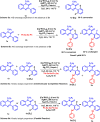
A rhodium-catalysed, regioselective synthetic methodology for selenylation and sulfenylation of 3-phenyl quinoxolinones has been developed through N-directed C-H activation in the presence of silver triflimide, and silver carbonate using dichalcogenides 'on water'. The methodology has been proven to be efficient, regioselective and green. Using this method, a range of selenylations and sulfenylations of the substrates has been carried out in good to excellent yields. Further, late-stage functionalisation produced potential anti-tumour, anti-fungal and anti-bacterial agents making these compounds potential drug candidates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


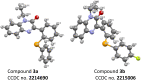


Similar articles
-
Efficient silver-catalyzed direct sulfenylation and selenylation of rich arenes.Org Biomol Chem. 2014 Dec 21;12(47):9557-61. doi: 10.1039/c4ob01992j. Epub 2014 Oct 21. Org Biomol Chem. 2014. PMID: 25333205
-
Electrochemical Regioselective C(sp2)-H Selenylation and Sulfenylation of Substituted 2-Amino-1,4-naphthoquinones.J Org Chem. 2023 Jan 20;88(2):1049-1060. doi: 10.1021/acs.joc.2c02486. Epub 2023 Jan 4. J Org Chem. 2023. PMID: 36599149
-
Copper-Catalyzed Sulfenylation, Sulfonylation, and Selenylation of 2,3-Allenoic Acids with Disulfides or Diselenides.J Org Chem. 2018 Jun 1;83(11):6101-6109. doi: 10.1021/acs.joc.8b00858. Epub 2018 May 23. J Org Chem. 2018. PMID: 29771529
-
Expedient, regioselective C-H chalcogenation of 3,4-dihydro-1,4-benzoxazines using a palladium-copper catalyst.Org Biomol Chem. 2024 Jul 17;22(28):5809-5815. doi: 10.1039/d4ob00524d. Org Biomol Chem. 2024. PMID: 38946460
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
Cited by
-
Visible-Light-Driven Regioselective Decarboxylative Acylation of N-Methyl-3-phenylquinoxalin-2(1H)-one by Dual Palladium-Photoredox Catalysis Through C-H Activation.ACS Omega. 2023 Dec 16;9(1):651-657. doi: 10.1021/acsomega.3c06367. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38239288 Free PMC article.
-
Transition-Metal Catalyzed Synthesis of Pyrimidines: Recent Advances, Mechanism, Scope and Future Perspectives.Top Curr Chem (Cham). 2024 Jan 31;382(1):4. doi: 10.1007/s41061-024-00451-2. Top Curr Chem (Cham). 2024. PMID: 38296918 Review.
References
-
- Sheldon R. A. Green Chem. 2005;7:267.
- Ballini R. Barboni L. Fringuelli F. Palmieri A. Pizzo F. Vaccaro L. Green Chem. 2007;9:823.
- Li C. J. Trost B. M. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13197. - PMC - PubMed
- Horváth I. T. Anastas P. T. Chem. Rev. 2007;107:2169. - PubMed
- Breslow R., The Principles and Reasons for Using Water as a Solvent for Green Chemistry, Handbook of Green Chemistry, 2010, pp. 1–29
- Katritzky A. R., Comprehensive Heterocyclic Chemistry III, Elsevier, Amsterdam, NY, 2008
- Brahmachari G., Green Synthetic Approaches for Biologically Relevant Heterocycles, Elsevier, Amsterdam, 2015, pp. 185–208
- Ameta K. L. and Dandia A., Green Chemistry: Synthesis of Bioactive Heterocycles, Springer, 2014
-
- Simon M. O. Li C. J. Chem. Soc. Rev. 2012;41:1415. - PubMed
- Constable D. J. C. Curzons A. D. Cunningham V. L. Green Chem. 2002;4:521.
-
- Li C. J. Chen L. Chem. Soc. Rev. 2006;35:68. - PubMed
- Li C. J. and Chan T. H., Comprehensive Organic Reactions in Aqueous Media, John Wiley and Sons, New York, 2007
- Dixneuf P. and Cadierno V., Metal-Catalyzed Reactions in Water, John Wiley & Sons, 2013
- Minakata S. Komastsu M. Chem. Rev. 2009;109:711. - PubMed
- Chanda A. Fokin V. V. Chem. Rev. 2009;109:725. - PMC - PubMed
-
- Rideout D. C. Breslow R. J. Am. Chem. Soc. 1980;102:7816.
- Moghaddam F. M. Hojabri L. Taheri S. Pirani P. J. Iran. Chem. Soc. 2010;7:781.
- Otto S. Boccaletti G. Engberts J. B. F. N. J. Am. Chem. Soc. 1998;120:4238.
- Liu X. Y. Che C. M. Angew. Chem., Int. Ed. 2008;47:3850. - PubMed
- Herrerias C. I. Yao X. Li Z. Li C. J. Chem. Rev. 2007;107:2546. - PubMed
- Lindstrom U. M. Andersson G. Angew. Chem., Int. Ed. 2006;45:548. - PubMed
- Zhou F. Li C. J. Nat. Commun. 2014;5:4254. - PubMed
-
- Narayan S. Muldoon J. Finn M. G. Fokin V. V. Kolb H. C. Sharpless K. B. Angew. Chem., Int. Ed. 2005;44:3275. - PubMed
LinkOut - more resources
Full Text Sources

