The potential role of the thymus in immunotherapies for acute myeloid leukemia
- PMID: 36814919
- PMCID: PMC9940763
- DOI: 10.3389/fimmu.2023.1102517
The potential role of the thymus in immunotherapies for acute myeloid leukemia
Abstract
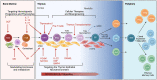
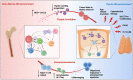
Understanding the factors which shape T-lymphocyte immunity is critical for the development and application of future immunotherapeutic strategies in treating hematological malignancies. The thymus, a specialized central lymphoid organ, plays important roles in generating a diverse T lymphocyte repertoire during the infantile and juvenile stages of humans. However, age-associated thymic involution and diseases or treatment associated injury result in a decline in its continuous role in the maintenance of T cell-mediated anti-tumor/virus immunity. Acute myeloid leukemia (AML) is an aggressive hematologic malignancy that mainly affects older adults, and the disease's progression is known to consist of an impaired immune surveillance including a reduction in naïve T cell output, a restriction in T cell receptor repertoire, and an increase in frequencies of regulatory T cells. As one of the most successful immunotherapies thus far developed for malignancy, T-cell-based adoptive cell therapies could be essential for the development of a durable effective treatment to eliminate residue leukemic cells (blasts) and prevent AML relapse. Thus, a detailed cellular and molecular landscape of how the adult thymus functions within the context of the AML microenvironment will provide new insights into both the immune-related pathogenesis and the regeneration of a functional immune system against leukemia in AML patients. Herein, we review the available evidence supporting the potential correlation between thymic dysfunction and T-lymphocyte impairment with the ontogeny of AML (II-VI). We then discuss how the thymus could impact current and future therapeutic approaches in AML (VII). Finally, we review various strategies to rejuvenate thymic function to improve the precision and efficacy of cancer immunotherapy (VIII).
Keywords: AML-acute myeloid leukemia; T lymphocytes; aging; hematopoietic (stem) cell transplant (HCST); immunosenescence; immunotherapy; thymus; tumor microenvironment.
Copyright © 2023 Hino, Xu, Xiao, Baylink, Reeves and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Thymic Function Associated With Cancer Development, Relapse, and Antitumor Immunity - A Mini-Review.Front Immunol. 2020 Apr 30;11:773. doi: 10.3389/fimmu.2020.00773. eCollection 2020. Front Immunol. 2020. PMID: 32425946 Free PMC article. Review.
-
Alterations of T-cell-mediated immunity in acute myeloid leukemia.Oncogene. 2020 Apr;39(18):3611-3619. doi: 10.1038/s41388-020-1239-y. Epub 2020 Mar 3. Oncogene. 2020. PMID: 32127646 Free PMC article. Review.
-
Immunosuppressive microenvironment in acute myeloid leukemia: overview, therapeutic targets and corresponding strategies.Ann Hematol. 2024 Dec;103(12):4883-4899. doi: 10.1007/s00277-024-06117-9. Epub 2024 Nov 28. Ann Hematol. 2024. PMID: 39607487 Review.
-
Molecular Markers of Regulatory T Cells in Cancer Immunotherapy with Special Focus on Acute Myeloid Leukemia (AML) - A Systematic Review.Curr Med Chem. 2020;27(28):4673-4698. doi: 10.2174/0929867326666191004164041. Curr Med Chem. 2020. PMID: 31584362
-
Immune-Based Therapeutic Interventions for Acute Myeloid Leukemia.Cancer Treat Res. 2022;183:225-254. doi: 10.1007/978-3-030-96376-7_8. Cancer Treat Res. 2022. PMID: 35551662
Cited by
-
Development of a Competitive Nutrient-Based T-Cell Immunotherapy Designed to Block the Adaptive Warburg Effect in Acute Myeloid Leukemia.Biomedicines. 2024 Oct 3;12(10):2250. doi: 10.3390/biomedicines12102250. Biomedicines. 2024. PMID: 39457563 Free PMC article.
-
Broadening the horizon: potential applications of CAR-T cells beyond current indications.Front Immunol. 2023 Nov 27;14:1285406. doi: 10.3389/fimmu.2023.1285406. eCollection 2023. Front Immunol. 2023. PMID: 38090582 Free PMC article. Review.
-
A Combination of the Immunotherapeutic Drug Anti-Programmed Death 1 with Lenalidomide Enhances Specific T Cell Immune Responses against Acute Myeloid Leukemia Cells.Int J Mol Sci. 2023 May 26;24(11):9285. doi: 10.3390/ijms24119285. Int J Mol Sci. 2023. PMID: 37298237 Free PMC article.
-
Imbalance of Th17 cells, Treg cells and associated cytokines in patients with systemic lupus erythematosus: a meta-analysis.Front Immunol. 2024 Jul 17;15:1425847. doi: 10.3389/fimmu.2024.1425847. eCollection 2024. Front Immunol. 2024. PMID: 39086480 Free PMC article.
-
Nutrient-gene therapy as a strategy to enhance CAR T cell function and overcome barriers in the tumor microenvironment.J Transl Med. 2025 Jun 6;23(1):633. doi: 10.1186/s12967-025-06606-z. J Transl Med. 2025. PMID: 40481543 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

