Characterization of acrosin and acrosin binding protein as novel CRISP2 interacting proteins in boar spermatozoa
- PMID: 36815564
- PMCID: PMC10947329
- DOI: 10.1111/andr.13413
Characterization of acrosin and acrosin binding protein as novel CRISP2 interacting proteins in boar spermatozoa
Abstract
Background: Previously, we reported that cysteine-rich secretory protein 2 is involved in high molecular weight complexes in boar spermatozoa. These cysteine-rich secretory protein 2protein complexes are formed at the last phase of sperm formation in the testis and play a role in sperm shaping and functioning.
Objectives: This study aimed to identify cysteine-rich secretory protein 2 interacting partners. These binding partner interactions were investigated under different conditions, namely, non-capacitating conditions, after the induction of in vitro sperm capacitation and subsequently during an ionophore A23187-induced acrosome reaction.
Materials and methods: The incubated pig sperm samples were subjected to protein extraction. Extracted proteins were subjected to blue native gel electrophoresis and native immunoblots. Immunoreactive gel bands were excised and subjected to liquid chromatography-mass spectrometry (LC-MS) analysis for protein identification. Protein extracts were also subjected to CRISP2 immunoprecipitation and analyzed by LC-MS for protein identification. The most prominent cystein-rich secretory protein 2 interacting proteins that appeared in both independent LC-MS analyses were studied with a functional in situ proximity interaction assay to validate their property to interact with cystein-rich secretory protein 2 in pig sperm.
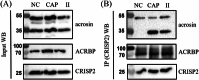
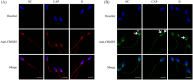
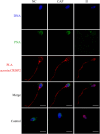
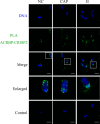
Results: Blue native gel electrophoresis and native immunoblots revealed that cystein-rich secretory protein 2 was present within a ∼150 kDa protein complex under all three conditions. Interrogation of cystein-rich secretory-protein 2-immunoreactive bands from blue native gels as well as cystein-rich secretory protein 2 immunoprecipitated products using mass spectrometry consistently revealed that, beyond cystein-rich secretory protein 2, acrosin and acrosin binding protein were among the most abundant interacting proteins and did interact under all three conditions. Co-immunoprecipitation and immunoblotting indicated that cystein-rich secretory protein 2 interacted with pro-acrosin (∼53 kDa) and Aacrosin binding protein under all three conditions and additionally to acrosin (∼35 kDa) after capacitation and the acrosome reaction. The colocalization of these interacting proteins with cystein-rich secretory protein 2 was assessed via in situ proximity ligation assays. The colocalization signal of cystein-rich secretory protein 2 and acrosin in the acrosome seemed dispersed after capacitation but was consistently present in the sperm tail under all conditions. The fluorescent foci of cystein-rich secretory protein 2 and acrsin binding protein colocalization appeared to be redistributed within the sperm head from the anterior acrosome to the post-acrosomal sheath region upon capacitation.
Discussion and conclusion: These results suggest that CRISP2 may act as a scaffold for protein complex formation and dissociation to ensure the correct positioning of proteins required for the acrosome reaction and zona pellucida penetration.
Keywords: CRISP2; boar spermatozoa; capacitation; colocalization; interacting proteins; protein complexes.
© 2023 The Authors. Andrology published by Wiley Periodicals LLC on behalf of American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors have no conflict of interests to disclose.
Figures


Similar articles
-
PH-20 but not acrosin is involved in sperm penetration of the macaque zona pellucida.Mol Reprod Dev. 1999 Jul;53(3):350-62. doi: 10.1002/(SICI)1098-2795(199907)53:3<350::AID-MRD11>3.0.CO;2-9. Mol Reprod Dev. 1999. PMID: 10369396
-
The serine protease testisin is present on the surface of capacitated stallion spermatozoa and interacts with key zona pellucida binding proteins.Andrology. 2019 Mar;7(2):199-212. doi: 10.1111/andr.12569. Epub 2018 Dec 13. Andrology. 2019. PMID: 30549223
-
Proteomic Characterization of Pig Sperm Anterior Head Plasma Membrane Reveals Roles of Acrosomal Proteins in ZP3 Binding.J Cell Physiol. 2015 Feb;230(2):449-63. doi: 10.1002/jcp.24728. J Cell Physiol. 2015. PMID: 25078272
-
Early steps of sperm-egg interactions during mammalian fertilization.Cell Biol Int. 1996 Jan;20(1):33-9. doi: 10.1006/cbir.1996.0006. Cell Biol Int. 1996. PMID: 8936405 Review.
-
MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization.Cell Tissue Res. 2012 Sep;349(3):881-95. doi: 10.1007/s00441-012-1429-1. Epub 2012 May 22. Cell Tissue Res. 2012. PMID: 22729485 Free PMC article. Review.
References
-
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168(4277):697‐698. - PubMed
-
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170(4321):326. - PubMed
-
- Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol. 2016;220:93‐106. - PubMed
-
- Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. Int J Dev Biol. 2008;52(5‐6):473‐480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

