Reduced Mortality among COVID-19 ICU Patients after Treatment with HemoClear Convalescent Plasma in Suriname
- PMID: 36815780
- PMCID: PMC10127603
- DOI: 10.1128/mbio.03379-22
Reduced Mortality among COVID-19 ICU Patients after Treatment with HemoClear Convalescent Plasma in Suriname
Abstract
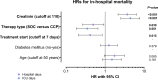
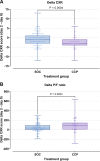
Convalescent plasma is a promising therapy for coronavirus disease 2019 (COVID-19), but its efficacy in intensive care unit (ICU) patients in low- and middle-income country settings such as Suriname is unknown. Bedside plasma separation using the HemoClear device made convalescent plasma therapy accessible as a treatment option in Suriname. Two hundred patients with severe SARS-CoV-2 infection requiring intensive care were recruited. Fifty eight patients (29%) received COVID-19 convalescent plasma (CCP) treatment in addition to standard of care (SOC). The CCP treatment and SOC groups were matched by age, sex, and disease severity scores. Mortality in the CCP treatment group was significantly lower than that in the SOC group (21% versus 39%; Fisher's exact test P = 0.0133). Multivariate analysis using ICU days showed that CCP treatment reduced mortality (hazard ratio [HR], 0.35; 95% confidence interval [CI], 0.18 to 0.66; P = 0.001), while complication of acute renal failure (creatinine levels, >110 mol/L; HR, 4.45; 95% CI, 2.54 to 7.80; P < 0.0001) was independently associated with death. Decrease in chest X-ray score in the CCP treatment group (median -3 points, interquartile range [IQR] -4 to -1) was significantly greater than that in the SOC group (median -1 point, IQR -3 to 1, Mann-Whitney test P = 0.0004). Improvement in the PaO2/FiO2 ratio was also significantly greater in the CCP treatment group (median 83, IQR 8 to 140) than in the SOC group (median 35, IQR -3 to 92, Mann-Whitney P = 0.0234). Further research is needed for HemoClear-produced CCP as a therapy for SARS-CoV-2 infection together with adequately powered, randomized controlled trials. IMPORTANCE This study compares mortality and other endpoints between intensive care unit COVID-19 patients treated with convalescent plasma plus standard of care (CCP), and a control group of patients hospitalized in the same medical ICU facility treated with standard of care alone (SOC) in a low- and middle-income country (LMIC) setting using bedside donor whole blood separation by gravity (HemoClear) to produce the CCP. It demonstrates a significant 65% survival improvement in HemoClear-produced CCP recipients (HR, 0.35; 95% CI, 0.19 to 0.66; P = 0.001). Although this is an exploratory study, it clearly shows the benefit of using the HemoClear-produced CCP in ICU patients in the Suriname LMIC setting. Additional studies could further substantiate our findings and their applicability for both LMICs and high-income countries and the use of CCP as a prepared readiness method to combat new viral pandemics.
Keywords: COVID-19; HemoClear; ICU; SARS-CoV-2; convalescent plasma; mortality.
Conflict of interest statement
The authors declare a conflict of interest. A. P. N. is the inventor of the HemoClear filter and holds stock ownership in HemoClear BV, Dr. Stolteweg 70, 8025 AZ Zwolle, The Netherlands. No involvement in actual patient treatment in Suriname. All other authors report no conflicts of interest.
Figures


References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi:10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, Yuan M-L, Zhang Y-L, Dai F-H, Liu Y, Wang Q-M, Zheng J-J, Xu L, Holmes EC, Zhang Y-Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi:10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, Varkey JB, Mehta AK, Lyon GM, Friedman-Moraco RJ, Marconi VC, Hill CE, Sullivan JN, Johnson DW, Lisco SJ, Mulligan MJ, Uyeki TM, McElroy AK, Sealy T, Campbell S, Spiropoulou C, Ströher U, Crozier I, Sacra R, Connor MJ, Sueblinvong V, Franch HA, Smith PW, Ribner BS, Nebraska Biocontainment Unit and the Emory Serious Communicable Diseases Unit . 2015. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis 61:496–502. doi:10.1093/cid/civ334. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

