Application of Genomic Sequencing to Refine Patient Stratification for Adjuvant Therapy in Renal Cell Carcinoma
- PMID: 36815791
- PMCID: PMC10068441
- DOI: 10.1158/1078-0432.CCR-22-1936
Application of Genomic Sequencing to Refine Patient Stratification for Adjuvant Therapy in Renal Cell Carcinoma
Abstract
Purpose: Patients with resected localized clear-cell renal cell carcinoma (ccRCC) remain at variable risk of recurrence. Incorporation of biomarkers may refine risk prediction and inform adjuvant treatment decisions. We explored the role of tumor genomics in this setting, leveraging the largest cohort to date of localized ccRCC tissues subjected to targeted gene sequencing.
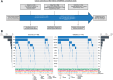
Experimental design: The somatic mutation status of 12 genes was determined in 943 ccRCC cases from a multinational cohort of patients, and associations to outcomes were examined in a Discovery (n = 469) and Validation (n = 474) framework.
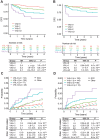
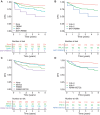
Results: Tumors containing a von-Hippel Lindau (VHL) mutation alone were associated with significantly improved outcomes in comparison with tumors containing a VHL plus additional mutations. Within the Discovery cohort, those with VHL+0, VHL+1, VHL+2, and VHL+≥3 tumors had disease-free survival (DFS) rates of 90.8%, 80.1%, 68.2%, and 50.7% respectively, at 5 years. This trend was replicated in the Validation cohort. Notably, these genomically defined groups were independent of tumor mutational burden. Amongst patients eligible for adjuvant therapy, those with a VHL+0 tumor (29%) had a 5-year DFS rate of 79.3% and could, therefore, potentially be spared further treatment. Conversely, patients with VHL+2 and VHL+≥3 tumors (32%) had equivalent DFS rates of 45.6% and 35.3%, respectively, and should be prioritized for adjuvant therapy.
Conclusions: Genomic characterization of ccRCC identified biologically distinct groups of patients with divergent relapse rates. These groups account for the ∼80% of cases with VHL mutations and could be used to personalize adjuvant treatment discussions with patients as well as inform future adjuvant trial design.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- 1078-0432. doi: 10.1158/1078-0432.CCR-29-7-HI doi: 10.1158/1078-0432.CCR-29-7-HI
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. - PubMed
-
- Dabestani S, Beisland C, Stewart GD, Bensalah K, Gudmundsson E, Lam TB, et al. Long-term outcomes of follow-up for initially localized clear- cell renal cell carcinoma: RECUR database analysis. Eur Urol Focus 2019;5:857–66. - PubMed
-
- Larroquette M, Peyraud F, Domblides C, Lefort F, Bernhard JC, Ravaud A, et al. Adjuvant therapy in renal cell carcinoma: current knowledge and future perspectives. Cancer Treat Rev 2021;97:102207. - PubMed
-
- Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal cell carcinoma. N Engl J Med 2021;385:683–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

