Eldecalcitol effectively prevents alveolar bone loss by partially improving Th17/Treg cell balance in diabetes-associated periodontitis
- PMID: 36815882
- PMCID: PMC9936814
- DOI: 10.3389/fbioe.2023.1070117
Eldecalcitol effectively prevents alveolar bone loss by partially improving Th17/Treg cell balance in diabetes-associated periodontitis
Abstract
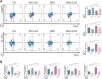
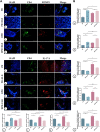
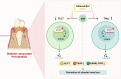
Background: Diabetes-associated periodontitis (DPD) is an inflammatory and destructive disease of periodontal tissues in the diabetic population. The disease is manifested as more severe periodontal destruction and is more difficult to treat when compared with periodontitis (PD). Eldecalcitol (ELD) is a novel active vitamin D3 analog; however, little clinical evidence is available on its role on improving PD and DPD, and its specific mechanisms remain unclear. In this study, we evaluated the preventative effects of ELD toward PD and DPD and explored its underlying molecular mechanisms. Methods: Experimental PD and DPD mouse models were established by ligation combined with lipopolysaccharide (LPS) from Porphyromonas gingivalis injection in C57BL/6J and C57BLKS/J Iar- + Leprdb/+Leprdb (db/db) mice, respectively. Simultaneously, ELD (0.25 μg/kg) was orally administered to mice via an intragastric method. Micro-computed tomography (CT), hematoxylin-eosin (HE) staining, immunohistochemistry (IHC), and tartrate-resistant acid phosphatase (TRAP) staining were used to evaluate alveolar bone alterations in vivo. Flow cytometry, immunofluorescence, and real-time polymerase chain reaction (qRT-PCR) were also used to examine gene expression and probe systemic and local changes in Treg and Th17 cell numbers. Additionally, western blotting and immunofluorescence staining were used to examine changes in STAT3/STAT5 signaling. Results: Micro-CT and HE staining showed that the DPD group had higher alveolar bone loss when compared with the PD group. After applying ELD, alveolar bone loss decreased significantly in both PD and DPD groups, and particularly evident in the DPD group. IHC and TRAP staining also showed that ELD promoted osteoblast activity while inhibiting the number of osteoclasts, and after ELD treatment, the receptor activator of nuclear factor-κB ligand (RANKL) to osteoprotegerin (OPG) ratio decreased. More importantly, this decreasing trend was more obvious in the DPD group. Flow cytometry and qRT-PCR also showed that the systemic Th17/Treg imbalance in PD and DPD groups was partially resolved when animals were supplemented with ELD, while immunofluorescence staining and qRT-PCR data showed the Th17/Treg imbalance was partially resolved in the alveolar bone of both ELD supplemented groups. Western blotting and immunofluorescence staining showed increased p-STAT5 and decreased p-STAT3 levels after ELD application. Conclusion: ELD exerted preventative effects toward PD and DPD by partially rectifying Th17/Treg cell imbalance via STAT3/STAT5 signaling. More importantly, given the severity of DPD, we found ELD was more advantageous in preventing DPD.
Keywords: STAT3/STAT5 signaling; Th17/Treg balance; diabetes-associated periodontitis; eldecalcitol; periodontitis.
Copyright © 2023 Gao, Zhang, Jiang, Zhai, Yu, Liu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

