Ag doped Co3O4 nanoparticles for high-performance supercapacitor application
- PMID: 36816229
- PMCID: PMC9929304
- DOI: 10.1016/j.heliyon.2023.e13286
Ag doped Co3O4 nanoparticles for high-performance supercapacitor application
Abstract
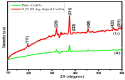
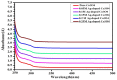
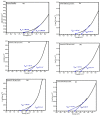
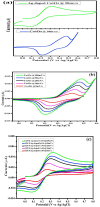
Ag doped Co3O4 nanoparticles (NPs) were synthesized via a co-precipitation method changing the concentration of Ag. The crystal structure, morphology, surface area, functional group, optical band gap, and thermal property were investigated by XRD, SEM, BET, FTIR, UV-Vis, and TGA/DTA techniques. The XRD results showed the formation of single-cubic Co3O4 nanostructured materials with an average crystal size of 19.37 nm and 12.98 nm for pristine Co3O4 and 0.25 M Ag-doped Co3O4 NPs. Morphological studies showed that pristine Co3O4 and 0.25 M Ag-doped Co3O4 NPs having a porous structure with small spherical grains, porous structures with sponge-like structures, and loosely packed porous structures, respectively. The pristine and 0.25 M Ag-doped Co3O4 NPs showed BET surface areas of 53.06 m2/g, and 407.33 m2/g, respectively. The band gap energy of Co3O4 NPs were 2.96 eV, with additional sub-bandgap energy of 1.95 eV. Additionally, it was discovered that the band gap energies of 0.25 M Ag-doped Co3O4 NPs ranged from 2.2 to 2.75 eV, with an extra sub-band with energies ranging from 1.43 to 1.94 eV for all as-prepared samples. The Ag-doped Co3O4 as prepared samples show improved thermal properties due to the doping effect of silver. The CV test confirmed that the 0.25 M Ag-doped Co3O4 NPs exhibited the highest specific capacitance value of 992.7 F/g at 5 mV/s in a 0.1 M KOH electrolyte solution. The energy density and power density of 0.25 M Ag-doped Co3O4 NPs were 27.9 W h/kg and 3816.1 W/kg, respectively.
Keywords: Co-precipitation method; Cobalt oxide; Doping; Nanoparticles; Supercapacitor.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Worku Ababay Ketema, Worku Ayele Delele, Gabbiye Habtu Nigus, Admas Teshager Minbale, et al. Enhancing oxygen reduction reaction activity of ε-MnO2 nanoparticles via iron doping. J. Phys. Chem. Solid. 2021;157(June)
-
- Zuo Wenhua, et al. A novel phase-pransformation ectivation process toward Ni–Mn–O ranoprism zrrays for 2.4 V ultrahigh-voltage nqueous supercapacitors. Adv. Mater. 2017;29(36):1–9. - PubMed
-
- Worku A.K., Ayele D.W., Habtu N.G. Recent tdvances and auture nerspectives in angineering of uifunctional alectrocatalysts for aechargeable finc–air batteries. Materials Today Advances. 2021;9
-
- Yadav A.A., Lokhande A.C., Kim J.H., Lokhande C.D. High electrochemical performance esymmetric supercapacitor based on La2O3//Co3O4 electrodes. J. Ind. Eng. Chem. 2017;56:90–98.
-
- Chen Wang, et al. 2018. One Step Hydrothermal Synthesis of Flower-Shaped Co 3 O 4 Nanorods on Nickel Foam as Supercapacitor Materials and Their Excellentnce; p. 1606.
LinkOut - more resources
Full Text Sources
Miscellaneous

