Formulation and Evaluation of Pravastatin Sodium-Loaded PLGA Nanoparticles: In vitro-in vivo Studies Assessment
- PMID: 36816332
- PMCID: PMC9936887
- DOI: 10.2147/IJN.S394701
Formulation and Evaluation of Pravastatin Sodium-Loaded PLGA Nanoparticles: In vitro-in vivo Studies Assessment
Abstract
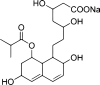
Purpose: Pravastatin sodium (PVS) is a hypolipidemic drug which suffers from extensive first-pass metabolism and short half-life. Poly(d,l-lactide-co-glycolide) (PLGA) is considered a promising carrier to improve its hypolipidemic and hepatoprotective activities.
Methods: PVS-loaded PLGA nanoparticles (PVS-PLGA-NPs) were prepared by double emulsion method using a full 32 factorial design. The in vitro release and the physical stability studies of the optimized PVS-PLGA-NPs (F5) were performed. Finally, both hypolipidemic and hepatoprotective activities of the optimized F5 NPs were studied and compared to PVS solution.
Results: All the studied physical parameters of the prepared NPs were found in the accepted range. The particle size (PS) ranged from 90 ± 0.125 nm to 179.33 ± 4.509 nm, the poly dispersity index (PDI) ranged from 0.121 ± 0.018 to 0.158 ± 0.014. The optimized NPs (F5) have the highest entrapment efficiency (EE%) (51.7 ± 5%), reasonable PS (168.4 ± 2.506 nm) as well as reasonable zeta potential (ZP) (-28.3 ± 1.18mv). Solid-state characterization indicated that PVS is well entrapped into NPs. All NPs have distinct spherical shape with smooth surface. The prepared NPs showed a controlled release profile. F5 showed good stability at 4 ± 2°C during the whole storage period of 3 months. In vivo study and histopathological examination indicated that F5 NPs showed significant increase in PVS hypolipidemic as well as hepatoprotective activity compared to PVS solution.
Conclusion: The PVS-PLGA-NPs could be considered a promising model to evade the first-pass effect and showed improvement in the hypolipidemic and hepatoprotective activities compared to PVS solution.
Keywords: PLGA; hypolipidemic and hepatoprotective activity; nanoparticles; pravastatin sodium.
© 2023 Elsayed et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures










References
-
- Castañeda PS, Escobar-Chávez JJ, Vázquez JA, Cruz IMR, Contreras LMM. Pravastatin transdermal patches: effect of the formulation and two different lengths of microneedles on in-vitro percutaneous absorption studies. Iran J Pharm Res. 2020;19(2):127–133. doi: 10.22037/ijpr.2019.1100914 - DOI - PMC - PubMed
-
- Campos‐Lara M, Pinto‐Almazán R, Oropeza MV, Mendoza‐Espinoza JA. Optimization of a pravastatin quantification method using HPLC with ultraviolet detection in human serum for monitoring dyslipidemic patients. J Liq Chromatogr. 2008;31(5):667–674. doi: 10.1080/10826070701853784 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

