Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor β antibody, in moderate-to-severe prurigo nodularis: a randomised, double-blind, placebo-controlled, phase 2a study
- PMID: 36816342
- PMCID: PMC9932343
- DOI: 10.1016/j.eclinm.2023.101826
Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor β antibody, in moderate-to-severe prurigo nodularis: a randomised, double-blind, placebo-controlled, phase 2a study
Abstract
Background: Prurigo nodularis is a chronic skin disease characterised by intensely pruritic, hyperkeratotic nodules. Vixarelimab, a human monoclonal antibody, binds to the beta subunit of the oncostatin M receptor, inhibiting signalling of both interleukin 31 and oncostatin M, two cytokine pathways that contribute to pruritus and nodule formation in prurigo nodularis.
Methods: This double-blind, placebo-controlled, phase 2a trial was done at both private and academic dermatology outpatient research clinics across the United States and Canada (n = 40). Patient eligibility criteria included age 18-75 years, physician-documented diagnosis of prurigo nodularis minimum 6 months duration of prurigo nodularis, presence of at least 10 pruritic nodules approximately 0.5-2 cm in size on at least two different anatomical locations (excluding face and scalp) and involving the extremities, and presence of normal-appearing skin between nodules; atopic dermatitis as a comorbidity was exclusionary. Patients were required to have moderate-to-severe pruritus, defined as Worst Itch-Numeric Rating Scale (WI-NRS) score ≥7 at screening and LS-mean weekly WI-NRS score ≥5 for each of the 2 consecutive weeks immediately before randomisation. Participants were randomly assigned (1:1) to receive weekly subcutaneous vixarelimab 360 mg (720 mg loading dose) or placebo using stratification factors (sex and presence of atopy) and block size 4 through the IWRS system. Stratification by atopy status was based on the reported high prevalence of atopy in this population and the potential impact of atopy in the immunopathologic process in prurigo nodularis. Patients, investigators, study sponsor, and site staff were masked to study treatment. The primary efficacy endpoint was least squares (LS)-mean percent change from baseline (PCFB) at Week 8 in weekly average Worst Itch-Numeric Rating Scale (WI-NRS) score. The primary efficacy endpoint was analysed with ANCOVA including treatment as fixed effect, with baseline WI-NRS, and randomisation stratification factor as covariates. All randomised patients who had at least 1 dose of study drug or placebo were included in the Safety Analysis Set. This trial is registered at ClinicalTrials.gov, NCT03816891.
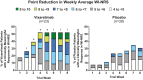
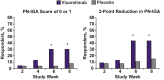
Findings: Of 50 patients randomised between March 11, 2019 and January 31, 2020, 23 vixarelimab recipients and 26 placebo recipients comprised the modified intent-to-treat analysis population (baseline LS-mean [SD] WI-NRS score, 8.3 [1.05]). Outcomes at Week 8 for vixarelimab versus placebo included LS-mean PCFB in WI-NRS score, -50.6% versus -29.4% (LS-mean difference [95% CI], -21.2% [-40.82, -1.60]; p = 0.03); ≥4-point reduction in WI-NRS score, 52.2% (12/23) versus 30.8% (8/26) (p = 0.11); PN-IGA score of 0 or 1, 30.4% (7/23) versus 7.7% (2/26) (p = 0.03); LS-mean PCFB in pruritus VAS score, -54.4% versus -32.6% (p = 0.03); and LS-mean PCFB sleep loss reduction (improvement), -56.3% versus -30.0% (p = 0.02). No deaths, serious TEAEs, or TEAEs leading to dose interruption were reported. The percentage of vixarelimab recipients reporting any TEAE was 91.3% (21/23) versus 76.9% (20/26) of placebo recipients; drug-related TEAEs generally were similar between the two groups (vixarelimab, 43.5% [10/23]; placebo, 38.5% [10/26]).
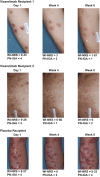
Interpretation: Vixarelimab demonstrated rapid reduction of pruritus and achievement of clear/almost clear skin in one-third of the patients by Week 8. Relief of itch and clearing of skin nodules represent two important potential therapeutic advances in the management of patients suffering from the debilitating disease Prurigo Nodularis.
Funding: Kiniksa Pharmaceuticals, Ltd.
Keywords: IL-31; Interleukin-31 receptor alpha; Nodular prurigo; Oncostatin M receptor beta; Prurigo nodularis; Pruritus; Vixarelimab.
© 2023 The Author(s).
Conflict of interest statement
HS has received honoraria, grants, and/or research funding as a speaker, investigator, and advisory board member for AbbVie, Amgen, BMS, Dermavant, Eli Lilly, Galderma, LEO Pharma, Kiniksa, and UCB. RB is an advisory board member, consultant, speaker, and/or investigator for, and receives honoraria and/or grants from, AbbVie, Arcutis, Arena Pharma, Asana Biosciences, Bellus Health, Bluefin Biomedicine, Boehringer-Ingelheim, Boston, CARA Therapeutic, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GlaxoSmithKline, Incyte, Inmagene Bio, Janssen, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Pfizer, Ralexar, RAPT Therapeutic, Regeneron, Respivant, Sanofi, Sienna, Target RWE, and Vyne Therapeutics. He is also an employee and shareholder of Innovaderm Research. GY is an advisory board member for Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, Trevi Therapeutics, Abbvie, Aslan and Arcutis; has received grants/research funding from Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Escient and Eli Lilly; and is an investigator for Regeneron Pharmaceuticals, Inc. and Sanofi. JS has received honoraria as a consultant and/or advisory board member for Abbvie, Afyx, Aobiome, Arena, Asana, Aslan, BioMX, Bluefin, Bodewell, Boehringer-Ingelheim, Celgene, Connect Biopharma, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Luna, Menlo, Novartis, Pfizer, RAPT, Regeneron, and Sanofi-Genzyme and as a speaker for Abbvie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; his institution has received grants from Galderma and Pfizer. ST has received grant support from Kiniksa for clinical research. WJL serves on speakers bureaus for, and receives honoraria from, Pfizer, Novartis, Amgen, Galderma Canada Inc., Janssen-Ortho, Leo Pharma, Abbvie, Hoffmann-La Roche Limited, Celgene, Pediapharm, and Bausch Health, and serves on advisory boards for Novartis, Janssen-Ortho, Amgen, Abbvie, Galderma, Leo Pharma, Sanofi Genzyme, Pfizer, Sun Pharma, Bausch Health, and Eli Lilly. MZ has received grants and research support from Pfizer, Novartis, Sienna, Janssen, Endo International, Lilly, Dermira, Moberg Pharma, Soligenix, Allergan, Asana BioSciences, Athenex, Foamix, Incyte, Sun Pharma and Verrice. ML has received grants and research support from AbbVie, Boehringer Ingelheim, Novartis, Lilly, Janssen, Leo Pharmaceuticals, Dermira, UCB, Aclaris, Valeant and Asana BioSciences. JFP is an employee of Kiniksa and owns stock in the company. GLJ and LZ are former employees of Kiniksa and were employed by the company at the time of the study.
Figures


References
-
- Zeidler C., Tsianakas A., Pereira M., Ständer H., Yosipovitch G., Ständer S. Chronic prurigo of nodular type: a review. Acta Derm Venereol. 2018;98(2):173–179. - PubMed
-
- Williams K.A., Roh Y.S., Brown I., et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67–77. - PubMed
-
- Elmariah S., Kim B., Berger T., et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747–760. - PubMed
-
- Steinke S., Zeidler C., Riepe C., et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: results of the European academy of dermatology and venereology network on assessment of severity and burden of pruritus (PruNet) cross-sectional trial. J Am Acad Dermatol. 2018;79(3):457–463. e5. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

