Adjuvant icotinib versus observation in patients with completely resected EGFR-mutated stage IB NSCLC (GASTO1003, CORIN): a randomised, open-label, phase 2 trial
- PMID: 36816343
- PMCID: PMC9932314
- DOI: 10.1016/j.eclinm.2023.101839
Adjuvant icotinib versus observation in patients with completely resected EGFR-mutated stage IB NSCLC (GASTO1003, CORIN): a randomised, open-label, phase 2 trial
Abstract
Background: This phase 2 trial aimed to compare adjuvant icotinib with observation in patients with epidermal growth factor receptor (EGFR) mutation-positive resected stage IB non-small cell lung cancer (NSCLC).
Methods: We performed a randomised, open-label, phase 2 trial from May 1, 2015 to December 29, 2020 at Sun Yat-sen University Cancer Center in China. Patients with completely resected, EGFR-mutant, stage IB (the 7th edition of TNM staging) NSCLC without adjuvant chemotherapy were randomised (1:1) to receive adjuvant therapy with icotinib (125 mg, three times daily) for 12 months or to undergo observation until disease progression or intolerable toxicity occurred. The primary endpoint was 3-year disease-free survival (DFS). CORIN (GASTO1003) was registered with Clinicaltrials.gov, with the number NCT02264210.
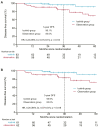
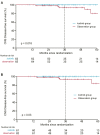
Findings: A total of 128 patients were randomised, with 63 patients in the icotinib group and 65 patients in the observation group. The median duration of follow-up was 39.9 months. The three-year DFS was significantly higher in the icotinib group (96.1%, 95% confidence interval [CI], 91.3-99.9) than in the observation group (84.0%, 95% CI, 75.1-92.9; P = 0.041). The DFS was significantly longer in the icotinib group than in the observation group, with a hazard ratio (HR) of 0.23 (95% CI, 0.07-0.81; P = 0.013). The OS data were immature, with three deaths in the observation arm. In the icotinib group, adverse events (AEs) of any grade were reported in 49 patients (77.8%), and grade 3 or greater AEs occurred in four patients (6.3%). No treatment-related deaths occurred.
Interpretation: Our findings suggested that adjuvant icotinib improved the 3-year DFS in patients with completely resected EGFR-mutated stage IB NSCLC with a manageable safety profile.
Funding: This study was sponsored by Betta Pharmaceutical Co., Ltd.
Keywords: Adjuvant therapy; Icotinib; Stage IB NSCLC.
© 2023 The Author(s).
Conflict of interest statement
LMD and ZLS are employees of Betta Pharmaceuticals Co., Ltd. TW and YP are employees of Hangzhou Repugene Technology Co., Ltd. Other co-authors declare no competing interests.
Figures


References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Datta D., Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–2103. - PubMed
-
- Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol. 2010;21(Suppl 7):vii196–v198. - PubMed
-
- Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

