Efficacy Evaluation of Bevacizumab Combined with Capecitabine in the Treatment of HER2-Negative Metastatic Breast Cancer: A Meta-Analysis
- PMID: 36816360
- PMCID: PMC9931458
- DOI: 10.1155/2023/8740221
Efficacy Evaluation of Bevacizumab Combined with Capecitabine in the Treatment of HER2-Negative Metastatic Breast Cancer: A Meta-Analysis
Abstract
Objective: This study aims to evaluate the efficacy of bevacizumab combined with capecitabine in treating HER2-negative metastatic breast cancer through meta-analysis.
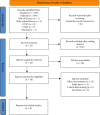
Methods: We searched literature from databases, including PubMed, Web of Science, Wiley Online Library, Ovid, CNKI, and Wanfang databases, for randomized controlled trials (RCTs) of bevacizumab combined with capecitabine (experimental group) and other treatments (control group) for HER2-negative metastatic breast cancer. Retrieved articles were published from the establishment of the database to August 9, 2022. The main outcome indicators were disease progression rate (RDP), disease progression-free survival (PFS), 1-year survival rate (OSR), the occurrence of serious adverse events (SAEs), and objective remission rate (ORR). The risk of bias was assessed according to the Cochrane systematic evaluation tool. Then, the meta-analysis was carried out using Stata16.0 software, and subgroup analysis was carried out based on various intervention methods in the control group.
Results: 8 RCTs were finally included in this study, including 2470 patients with HER2-negative metastatic breast cancer. The results of meta-analysis showed that bevacizumab combined with capecitabine had no significant advantage over the control group in terms of RDP, but the results of subgroup analysis were consistent and significant (subgroup 1 (bevacizumab or chemotherapy): DR = -0.03, 95% CI (-0.14, 0.09), P = 0.01; subgroup 2 (bevacizumab plus paclitaxel therapy): DR = -0.03, 95% CI (-0.14, 0.09), P = 0.03). Furthermore, there was no statistical difference in terms of PFS of the experimental group (MD = 9.24, 95% CI (7.88, 32.67), P = 0.05). However, the subgroup analysis showed that the combination of bevacizumab and capecitabine demonstrated a more significant significance than bevacizumab or chemotherapy alone (subgroup 1: MD = 10.11, 95% CI (7.88, 12.34), P = 0.00). Compared with the control group, the experimental group had significant differences in OSR (DR = 0.07, 95% CI (-0.01, 0.15), P = 0.00) and ORR (DR = 0.07, 95% CI (-0.01, 0.15), P = 0.00). In terms of safety, the incidence of serious adverse events in the experimental group did not show a statistically significant difference (MD = 0.01, 95% CI (-0.21, 0.19), P = 0.82). When subgroup analyses were performed, the bevacizumab plus capecitabine regimen was associated with an increased incidence of serious adverse events compared with the drug alone (subgroup 1: MD = 0.02, 95% CI (-0.16, 0.20), P = 0.00) but a reduction in serious adverse events compared with the bevacizumab plus paclitaxel regimen (subgroup 2: DR = -0.01, 95% CI (-0.21, 0.19), P = 0.00).
Conclusion: The combination therapy of bevacizumab and capecitabine can significantly improve the RDP and OSR of patients compared with the control group. The PFS and ORR of the experimental group are significantly higher than those of bevacizumab or chemotherapy alone. Still, no statistical difference was observed for these outcome indicators between two combined treatments of bevacizumab with capecitabine or paclitaxel. Although this combined treatment scheme may increase the incidence of serious adverse events compared with that of bevacizumab or chemotherapy alone, the incidence of adverse events was decreased compared with bevacizumab combined with paclitaxel. Therefore, the chemotherapy regimen for HER2-negative metastatic breast cancer in clinical practice can be selected according to the actual situation of the patients.
Copyright © 2023 Yiyi Hu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial.Lancet Oncol. 2016 Sep;17(9):1230-9. doi: 10.1016/S1470-2045(16)30154-1. Epub 2016 Aug 5. Lancet Oncol. 2016. PMID: 27501767 Clinical Trial.
-
Bevacizumab: a review of its use in combination with paclitaxel or capecitabine as first-line therapy for HER2-negative metastatic breast cancer.Drugs. 2011 Nov 12;71(16):2213-29. doi: 10.2165/11207720-000000000-00000. Drugs. 2011. PMID: 22035518 Review.
-
Paclitaxel and bevacizumab with or without capecitabine as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a multicentre, open-label, randomised phase 2 trial.Eur J Cancer. 2014 Dec;50(18):3077-88. doi: 10.1016/j.ejca.2014.10.008. Eur J Cancer. 2014. PMID: 25459393 Clinical Trial.
-
Efficacy of bevacizumab combined with chemotherapy in the treatment of HER2-negative metastatic breast cancer: a network meta-analysis.BMC Cancer. 2020 Mar 4;20(1):180. doi: 10.1186/s12885-020-6674-1. BMC Cancer. 2020. PMID: 32131770 Free PMC article.
-
[Efficacy of neoadjuvant chemotherapy combined with bevacizumab versus neoadjuvant chemotherapy alone for Her2-negative breast cancer: a meta-analysis of randomized controlled clinical trials].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(4):379-386. doi: 10.3785/j.issn.1008-9292.2016.07.08. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27868411 Free PMC article. Review. Chinese.
Cited by
-
Advancements in Understanding the Hide-and-Seek Strategy of Hibernating Breast Cancer Cells and Their Implications in Oncology from a Broader Perspective: A Comprehensive Overview.Curr Issues Mol Biol. 2024 Aug 1;46(8):8340-8367. doi: 10.3390/cimb46080492. Curr Issues Mol Biol. 2024. PMID: 39194709 Free PMC article. Review.
References
-
- Dong L., Zhu L. N., Xie B. J., et al. Comparative effectiveness of taxane‐containing regimens for treatment of HER2‐negative metastatic breast cancer: a network meta‐analysis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy . 2019;39(12):1126–1136. doi: 10.1002/phar.2344. - DOI - PubMed
-
- Bracarda S., Bellmunt J., Melichar B., et al. Overall survival in patients with metastatic renal cell carcinoma initially treated with bevacizumab plus interferon-α2a and subsequent therapy with tyrosine kinase inhibitors: a retrospective analysis of the phase III AVOREN trial. BJU International . 2011;107(2):214–219. doi: 10.1111/j.1464-410x.2010.09707.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

