Cell migration is impaired in XPA-deficient cells
- PMID: 36816512
- PMCID: PMC9927838
- DOI: 10.1096/fba.2022-00084
Cell migration is impaired in XPA-deficient cells
Abstract
Xeroderma pigmentosum (XP) is a hereditary disorder characterized by photosensitivity, predisposition to skin cancers, and neurological abnormalities including microcephaly and progressive neurodegeneration. A lack of nucleotide excision repair (NER) in patients with XP can cause hypersensitivity to the sun, leading to skin cancer, whereas the etiology of the neuronal symptoms of XP remains ambiguous. There are various neurological disorders that perturb neuronal migration, causing mislocalization and disorganization of the cortical lamination. Here, we investigated the role of the XP group-A (Xpa) gene in directed cell migration. First, we adopted an in utero electroporation method to transduce shRNA vectors into the murine embryonic cerebral cortex for the in vivo knockdown of Xpa. Xpa-knockdown neurons in the embryonic cerebral cortex showed abnormal cell migration, cell cycle exit, and differentiation. The genotype-phenotype relationship between the lack of XPA and cell migration abnormalities was confirmed using both a scratch assay and time-lapse microscopy in XP-A patient-derived fibroblasts. Unlike healthy cells, these cells showed impairment in overall mobility and the direction of motility. Therefore, abnormal cell migration may explain neural tissue abnormalities in patients with XP-A.
© 2022 The Authors. FASEB BioAdvances published by Wiley Periodicals LLC on behalf of The Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
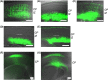



Similar articles
-
Mutational spectrum of Xeroderma pigmentosum group A in Egyptian patients.Gene. 2014 Jan 1;533(1):52-6. doi: 10.1016/j.gene.2013.09.125. Epub 2013 Oct 14. Gene. 2014. PMID: 24135642
-
UV-induced skin carcinogenesis in xeroderma pigmentosum group A (XPA) gene-knockout mice with nucleotide excision repair-deficiency.Mutat Res. 2001 Jun 2;477(1-2):31-40. doi: 10.1016/s0027-5107(01)00093-8. Mutat Res. 2001. PMID: 11376684
-
A disease-associated XPA allele interferes with TFIIH binding and primarily affects transcription-coupled nucleotide excision repair.Proc Natl Acad Sci U S A. 2023 Mar 14;120(11):e2208860120. doi: 10.1073/pnas.2208860120. Epub 2023 Mar 9. Proc Natl Acad Sci U S A. 2023. PMID: 36893274 Free PMC article.
-
Common pathways for ultraviolet skin carcinogenesis in the repair and replication defective groups of xeroderma pigmentosum.J Dermatol Sci. 2000 May;23(1):1-11. doi: 10.1016/s0923-1811(99)00088-2. J Dermatol Sci. 2000. PMID: 10699759 Review.
-
DNA repair-deficient Xpa and Xpa/p53+/- knock-out mice: nature of the models.Toxicol Pathol. 2001;29 Suppl:109-16. doi: 10.1080/019262301753178519. Toxicol Pathol. 2001. PMID: 11695546 Review.
Cited by
-
DNA damage and repair: underlying mechanisms leading to microcephaly.Front Cell Dev Biol. 2023 Oct 10;11:1268565. doi: 10.3389/fcell.2023.1268565. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37881689 Free PMC article. Review.
-
Therapeutic strategies targeting cellular senescence for cancer and other diseases.J Biochem. 2024 Apr 29;175(5):525-537. doi: 10.1093/jb/mvae015. J Biochem. 2024. PMID: 38366629 Free PMC article. Review.
References
-
- Ueda T, Kanda F, Nishiyama M, Nishigori C, Toda T. Quantitative analysis of brain atrophy in patients with xeroderma pigmentosum group a carrying the founder mutation in Japan. J Neurol Sci. 2017;381:103‐106. - PubMed
-
- Nishigori C, Nakano E, Masaki T, et al. Characteristics of Xeroderma Pigmentosum in Japan: lessons from two clinical surveys and measures for patient care. Photochem Photobiol. 2019;95(1):140‐153. - PubMed
-
- Nishigori C, Moriwaki S, Takebe H, Tanaka T, Imamura S. Gene alterations and clinical characteristics of xeroderma pigmentosum group a patients in Japan. Arch Dermatol. 1994;130(2):191‐197. - PubMed
LinkOut - more resources
Full Text Sources
