Effects of intensive vs. standard blood pressure control on cognitive function: Post-hoc analysis of the STEP randomized controlled trial
- PMID: 36816574
- PMCID: PMC9930906
- DOI: 10.3389/fneur.2023.1042637
Effects of intensive vs. standard blood pressure control on cognitive function: Post-hoc analysis of the STEP randomized controlled trial
Abstract
Background: The STEP (Strategy of Blood Pressure Intervention in the older Hypertensive Patients) trial showed that intensive systolic blood pressure (SBP) control resulted in a lower incidence of cardiovascular events than standard treatment. This study analyzed the effects of intensive SBP lowering on cognitive function.
Methods: STEP was a multicenter, randomized controlled trial of hypertensive patients aged 60-80 years. Participants were randomly assigned (1:1) to SBP goals of 110-130 mmHg (intensive treatment) or 130-150 mmHg (standard treatment). Each individual was asked to complete a cognitive function test (Mini-Mental State Examination; MMSE) at baseline and during follow-up. The primary outcome for this study was the annual change in MMSE score. Subjects with a score less than education-specific cutoff point were categorized as cognitive decline.
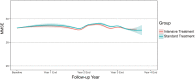
Results: The analysis enrolled 6,501 participants (3,270 participants in the intensive-treatment and 3,231 participants in the standard-treatment groups). Median follow-up was 3.34 years. There was a minor change in MMSE score, with an annual change of -0.001 [95% confidence interval [CI] -0.020, 0.018] and 0.030 (95% CI 0.011, 0.049) in the intensive- and standard-treatment groups, respectively (p = 0.052). Cognitive decline occurred in 46/3,270 patients (1.4%) in the intensive-treatment group and 42/3,231 (1.3%) in the standard-treatment group (hazard ratio 0.005, 95% CI 0.654, 1.543, p = 0.983).
Conclusions: Compared with standard treatment, intensive SBP treatment did not result in a significant change in cognitive function test score. The impact of intensive blood pressure lowering was not evident using this global cognitive function test.
Trial registration: ClinicalTrials.gov. Unique identifier: NCT03015311.
Keywords: Mini-Mental State Examination; cardiovascular events; cognitive function; intensive BP treatment; standard BP treatment.
Copyright © 2023 Fan, Bai, Liu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- National Academies of Sciences E Medicine Health Medicine D Board Board on Health Sciences P Committee Committee on Preventing D . In: Downey A, Stroud C, Landis S, Leshner AI, editors. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press (US). (2017). - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

