Regulatory effects of oral microbe on intestinal microbiota and the illness
- PMID: 36816583
- PMCID: PMC9928999
- DOI: 10.3389/fcimb.2023.1093967
Regulatory effects of oral microbe on intestinal microbiota and the illness
Abstract
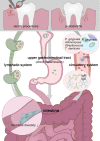
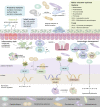
Over the past decade, the association between oral health, intestinal microbiota, and systemic diseases has been further validated. Some oral microbial species have been isolated from pathological intestine mucosa or feces and identified as biomarkers for intestinal diseases. A small proportion of oral microbiome passes through or colonizes the lower gastrointestinal tract, even in healthy individuals. Opportunistic pathogens from the oral cavity may expand and participate in the occurrence and progression of intestinal diseases when the anatomical barrier is disrupted. These disruptors interact with the intestinal microbiota, disturbing indigenous microorganisms, and mucosal barriers through direct colonization, blood circulation, or derived metabolite pathways. While interacting with the host's immune system, oral-derived pathogens stimulate inflammation responses and guide the transition of the intestinal microenvironment from a healthy state to a pre-disease state. Therefore, the oral-gut microbiome axis sheds light on new clinical therapy options, and gastrointestinal tract ecology balance necessitates simultaneous consideration of both oral and gut microbiomes. This review summarizes possible routes of oral microbes entering the intestine and the effects of certain oral bacteria on intestinal microbiota and the host's immune responses.
Keywords: gut diseases; immunity; intestinal microbe; microbiota; oral microbe; oral-gut axis.
Copyright © 2023 Lu, Li and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088
-
Maternal milk and fecal microbes guide the spatiotemporal development of mucosa-associated microbiota and barrier function in the porcine neonatal gut.BMC Biol. 2019 Dec 18;17(1):106. doi: 10.1186/s12915-019-0729-2. BMC Biol. 2019. PMID: 31852478 Free PMC article.
-
Succinate metabolism and its regulation of host-microbe interactions.Gut Microbes. 2023 Jan-Dec;15(1):2190300. doi: 10.1080/19490976.2023.2190300. Gut Microbes. 2023. PMID: 36946592 Free PMC article. Review.
-
Gut microbiota as a key regulator of intestinal mucosal immunity.Life Sci. 2024 May 15;345:122612. doi: 10.1016/j.lfs.2024.122612. Epub 2024 Apr 6. Life Sci. 2024. PMID: 38588949 Review.
-
The gut-liver axis in liver disease: Pathophysiological basis for therapy.J Hepatol. 2020 Mar;72(3):558-577. doi: 10.1016/j.jhep.2019.10.003. Epub 2019 Oct 14. J Hepatol. 2020. PMID: 31622696 Review.
Cited by
-
Gender-specific microbial signatures in saliva: Unveiling the association between the oral microbiome and the pathogenesis of glioma.Heliyon. 2024 Aug 31;10(17):e37284. doi: 10.1016/j.heliyon.2024.e37284. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296230 Free PMC article.
-
The interaction between oral microbiota and gut microbiota in atherosclerosis.Front Cardiovasc Med. 2024 Jun 12;11:1406220. doi: 10.3389/fcvm.2024.1406220. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38932989 Free PMC article. Review.
-
Oral Microbiome Dysbiosis as a Risk Factor for Stroke: A Comprehensive Review.Microorganisms. 2024 Aug 22;12(8):1732. doi: 10.3390/microorganisms12081732. Microorganisms. 2024. PMID: 39203574 Free PMC article. Review.
-
When inflammatory stressors dramatically change, disease phenotypes may transform between autoimmune hematopoietic failure and myeloid neoplasms.Front Immunol. 2024 Feb 15;15:1339971. doi: 10.3389/fimmu.2024.1339971. eCollection 2024. Front Immunol. 2024. PMID: 38426096 Free PMC article. Review.
-
Advancing vaccine technology through the manipulation of pathogenic and commensal bacteria.Mater Today Bio. 2024 Nov 16;29:101349. doi: 10.1016/j.mtbio.2024.101349. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39850273 Free PMC article. Review.
References
-
- Abed J., Maalouf N., Manson A. L., Earl A. M., Parhi L., Emgard J. E. M., et al. . (2020). Colon cancer-associated fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00400 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

