Modulating the activity of human nociceptors with a SCN10A promoter-specific viral vector tool
- PMID: 36816616
- PMCID: PMC9932673
- DOI: 10.1016/j.ynpai.2023.100120
Modulating the activity of human nociceptors with a SCN10A promoter-specific viral vector tool
Abstract
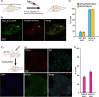
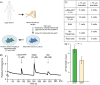
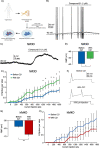
Despite the high prevalence of chronic pain as a disease in our society, there is a lack of effective treatment options for patients living with this condition. Gene therapies using recombinant AAVs are a direct method to selectively express genes of interest in target cells with the potential of, in the case of nociceptors, reducing neuronal firing in pain conditions. We designed a recombinant AAV vector expressing cargos whose expression was driven by a portion of the SCN10A (NaV1.8) promoter, which is predominantly active in nociceptors. We validated its specificity for nociceptors in mouse and human dorsal root ganglia and showed that it can drive the expression of functional proteins. Our viral vector and promoter package drove the expression of both excitatory or inhibitory DREADDs in primary human DRG cultures and in whole cell electrophysiology experiments, increased or decreased neuronal firing, respectively. Taken together, we present a novel viral tool that drives expression of cargo specifically in human nociceptors. This will allow for future specific studies of human nociceptor properties as well as pave the way for potential future gene therapies for chronic pain.
Keywords: AAV; Chemogenetics; DREADD; DRG; Gene therapy; Pain; Sodium channel.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F., et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

