Nonaqueous Li-Mediated Nitrogen Reduction: Taking Control of Potentials
- PMID: 36816775
- PMCID: PMC9926486
- DOI: 10.1021/acsenergylett.2c02697
Nonaqueous Li-Mediated Nitrogen Reduction: Taking Control of Potentials
Abstract
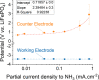
The performance of the Li-mediated ammonia synthesis has progressed dramatically since its recent reintroduction. However, fundamental understanding of this reaction is slower paced, due to the many uncontrolled variables influencing it. To address this, we developed a true nonaqueous LiFePO4 reference electrode, providing both a redox anchor from which to measure potentials against and estimates of sources of energy efficiency loss. We demonstrate its stable electrochemical potential in operation using different N2- and H2-saturated electrolytes. Using this reference, we uncover the relation between partial current density and potentials. While the counter electrode potential increases linearly with current, the working electrode remains stable at lithium plating, suggesting it to be the only electrochemical step involved in this process. We also use the LiFePO4/Li+ equilibrium as a tool to probe Li-ion activity changes in situ. We hope to drive the field toward more defined systems to allow a holistic understanding of this reaction.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Andersen S. Z.; Čolić V.; Yang S.; Schwalbe J. A.; Nielander A. C.; McEnaney J. M.; Enemark-Rasmussen K.; Baker J. G.; Singh A. R.; Rohr B. A.; Statt M. J.; Blair S. J.; Mezzavilla S.; Kibsgaard J.; Vesborg P. C. K.; Cargnello M.; Bent S. F.; Jaramillo T. F.; Stephens I. E. L.; Nørskov J. K.; Chorkendorff I. A Rigorous Electrochemical Ammonia Synthesis Protocol with Quantitative Isotope Measurements. Nature 2019, 570 (7762), 504–508. 10.1038/s41586-019-1260-x. - DOI - PubMed
-
- Tsuneto A.; Kudo A.; Sakata T. Lithium-Mediated Electrochemical Reduction of High Pressure N2 to NH3. J. Electroanal. Chem. 1994, 367 (1–2), 183–188. 10.1016/0022-0728(93)03025-K. - DOI
-
- Li S.; Zhou Y.; Li K.; Saccoccio M.; Sažinas R.; Andersen S. Z.; Pedersen J. B.; Fu X.; Shadravan V.; Chakraborty D.; Kibsgaard J.; Vesborg P. C. K.; Nørskov J. K.; Chorkendorff I. Electrosynthesis of Ammonia with High Selectivity and High Rates via Engineering of the Solid-Electrolyte Interphase. Joule 2022, 6 (9), 2083–2101. 10.1016/j.joule.2022.07.009. - DOI - PMC - PubMed
-
- Steinberg K.; Yuan X.; Klein C. K.; Lazouski N.; Mecklenburg M.; Manthiram K.; Li Y. Imaging of Nitrogen Fixation at Lithium Solid Electrolyte Interphases via Cryo-Electron Microscopy. Nat. Energy 2022, 1–11. 10.1038/s41560-022-01177-5. - DOI
-
- Westhead O.; Spry M.; Bagger A.; Shen Z.; Yadegari H.; Favero S.; Tort R.; Titirici M.; Ryan M. P.; Jervis R.; Katayama Y.; Aguadero A.; Regoutz A.; Grimaud A.; Stephens I. E. L. The Role of Ion Solvation in Lithium Mediated Nitrogen Reduction. J. Mater. Chem. A 2023, 10.1039/D2TA07686A. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
