Water Increases the Faradaic Selectivity of Li-Mediated Nitrogen Reduction
- PMID: 36816776
- PMCID: PMC9926485
- DOI: 10.1021/acsenergylett.2c02792
Water Increases the Faradaic Selectivity of Li-Mediated Nitrogen Reduction
Abstract
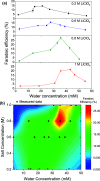
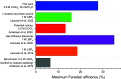
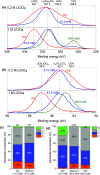
The lithium-mediated system catalyzes nitrogen to ammonia under ambient conditions. Herein we discover that trace amount of water as an electrolyte additive-in contrast to prior reports from the literature-can effect a dramatic improvement in the Faradaic selectivity of N2 reduction to NH3. We report that an optimal water concentration of 35.9 mM and LiClO4 salt concentration of 0.8 M allows a Faradaic efficiency up to 27.9 ± 2.5% at ambient pressure. We attribute the increase in Faradaic efficiency to the incorporation of Li2O in the solid electrolyte interphase, as suggested by our X-ray photoelectron spectroscopy measurements. Our results highlight the extreme sensitivity of lithium-mediated N2 reduction to small changes in the experimental conditions.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Tsuneto A.; Kudo A.; Sakata T. Lithium-mediated electrochemical reduction of high pressure N2 to NH 3. J. Electroanal. Chem. 1994, 367, 183–188. 10.1016/0022-0728(93)03025-K. - DOI
-
- Lazouski N.; Schiffer Z. J.; Williams K.; Manthiram K. Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule 2019, 3 (4), 1127–1139. 10.1016/j.joule.2019.02.003. - DOI
-
- Cherepanov P v.; Krebsz M.; Hodgetts R. Y.; Simonov A. N.; Macfarlane D. R. Understanding the Factors Determining the Faradaic Efficiency and Rate of the Lithium Redox-Mediated N2Reduction to Ammonia. J. Phys. Chem. C 2021, 125 (21), 11402–11410. 10.1021/acs.jpcc.1c02494. - DOI
LinkOut - more resources
Full Text Sources
