Real-world analysis of the use of lenvatinib in differentiated thyroid cancers
- PMID: 36816785
- PMCID: PMC9937066
- DOI: 10.3332/ecancer.2023.1500
Real-world analysis of the use of lenvatinib in differentiated thyroid cancers
Abstract
Introduction: Lenvatinib is one of the approved treatments for radioiodine-refractory differentiated thyroid cancers. However, there is very limited data from India on real-world efficacy and adverse events of Lenvatinib and hence this analysis was performed.
Methods: This was a retrospective analysis in which patients of radioiodine-refractory differentiated thyroid cancer as per the SELECT study criteria, who received lenvatinib, were selected for the study over the last 4 years. The baseline demographic characteristics, adverse events of lenvatinib, the date of progression and the date of overall survival (OS) were extracted from the electronic medical records of Tata Memorial Hospital. SPSS version 20 was used for analysis.
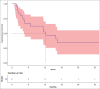
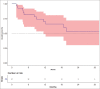
Results: The median starting dose of lenvatinib was 20 mg. Fifteen events for progression had occurred and the median progression-free survival (PFS) was 12.2 months [95% CI: 4.4-not available (NA)]. The events for OS analysis were 12. The median OS was 35.3 months (95% CI: 11.4-NA). There was no impact on starting dose on PFS or OS.
Conclusion: The real-world data of Lenvatinib suggest a lot of variability in the starting dose. In spite of this variability, the response rates and OS are similar to that noted in pivotal study. This suggests a case for need for more studies evaluating lower doses of Lenvatinib.
Keywords: dedifferentiated; follicular; lenvatinib; papillary; radioiodine refractory; thyroid cancer.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
None.
Figures
Similar articles
-
Efficacy and Limitations of Lenvatinib Therapy for Radioiodine-Refractory Differentiated Thyroid Cancer: Real-World Experiences.Thyroid. 2020 Feb;30(2):214-221. doi: 10.1089/thy.2019.0221. Epub 2020 Jan 23. Thyroid. 2020. PMID: 31854270
-
Low Dose of Lenvatinib Treatment for Patients of Radioiodine-Refractory Differentiated Thyroid Carcinoma - A Real-World Experience.Cancer Manag Res. 2021 Sep 14;13:7139-7148. doi: 10.2147/CMAR.S326255. eCollection 2021. Cancer Manag Res. 2021. PMID: 34548818 Free PMC article.
-
Extended Real-World Observation of Patients Treated with Sorafenib for Radioactive Iodine-Refractory Differentiated Thyroid Carcinoma and Impact of Lenvatinib Salvage Treatment: A Korean Multicenter Study.Thyroid. 2019 Dec;29(12):1804-1810. doi: 10.1089/thy.2019.0246. Epub 2019 Oct 8. Thyroid. 2019. PMID: 31592739
-
Lenvatinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer: Treatment Optimization for Maximum Clinical Benefit.Oncologist. 2022 Jul 5;27(7):565-572. doi: 10.1093/oncolo/oyac065. Oncologist. 2022. PMID: 35482606 Free PMC article. Review.
-
Lenvatinib - A multikinase inhibitor for radioiodine-refractory differentiated thyroid cancer.J Oncol Pharm Pract. 2018 Jan;24(1):28-32. doi: 10.1177/1078155216680119. Epub 2016 Nov 18. J Oncol Pharm Pract. 2018. PMID: 27856921 Review.
Cited by
-
Efficacy and Safety of Tyrosine Kinase Inhibitors in Downstaging and Palliation in Patients with Advanced Differentiated Thyroid Cancer - A Multicentre study.Indian J Surg Oncol. 2025 Feb;16(1):165-171. doi: 10.1007/s13193-024-02053-2. Epub 2024 Aug 9. Indian J Surg Oncol. 2025. PMID: 40114887
-
Real-world lenvatinib use in metastatic thyroid cancer: early dose intensity and side effect profile in an Australian centre.Endocr Oncol. 2025 Jun 19;5(1):e240062. doi: 10.1530/EO-24-0062. eCollection 2025 Jan. Endocr Oncol. 2025. PMID: 40556750 Free PMC article.
References
-
- Arance A, de la Cruz-Merino L, Petrella TM, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2022;2022:JCO2200221. - PubMed
LinkOut - more resources
Full Text Sources