Integration of structural MRI and epigenetic analyses hint at linked cellular defects of the subventricular zone and insular cortex in autism: Findings from a case study
- PMID: 36817099
- PMCID: PMC9935943
- DOI: 10.3389/fnins.2022.1023665
Integration of structural MRI and epigenetic analyses hint at linked cellular defects of the subventricular zone and insular cortex in autism: Findings from a case study
Abstract
Introduction: Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social interaction, communication and repetitive, restrictive behaviors, features supported by cortical activity. Given the importance of the subventricular zone (SVZ) of the lateral ventrical to cortical development, we compared molecular, cellular, and structural differences in the SVZ and linked cortical regions in specimens of ASD cases and sex and age-matched unaffected brain.
Methods: We used magnetic resonance imaging (MRI) and diffusion tractography on ex vivo postmortem brain samples, which we further analyzed by Whole Genome Bisulfite Sequencing (WGBS), Flow Cytometry, and RT qPCR.
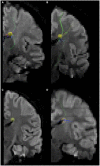
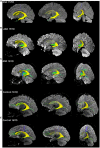
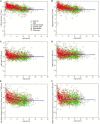
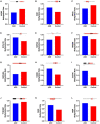
Results: Through MRI, we observed decreased tractography pathways from the dorsal SVZ, increased pathways from the posterior ventral SVZ to the insular cortex, and variable cortical thickness within the insular cortex in ASD diagnosed case relative to unaffected controls. Long-range tractography pathways from and to the insula were also reduced in the ASD case. FACS-based cell sorting revealed an increased population of proliferating cells in the SVZ of ASD case relative to the unaffected control. Targeted qPCR assays of SVZ tissue demonstrated significantly reduced expression levels of genes involved in differentiation and migration of neurons in ASD relative to the control counterpart. Finally, using genome-wide DNA methylation analyses, we identified 19 genes relevant to neurological development, function, and disease, 7 of which have not previously been described in ASD, that were significantly differentially methylated in autistic SVZ and insula specimens.
Conclusion: These findings suggest a hypothesis that epigenetic changes during neurodevelopment alter the trajectory of proliferation, migration, and differentiation in the SVZ, impacting cortical structure and function and resulting in ASD phenotypes.
Keywords: DNA methylation; MRI; SVZ; autism; cortex; epigenetic.
Copyright © 2023 Takahashi, Allan, Peres, Ortug, van der Kouwe, Valli, Ethier, Levman, Baumer, Tsujimura, Vargas-Maya, McCracken, Lee and Maunakea.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5, 5th Edn. Arlington, VA: American Psychiatric Association.
Grants and funding
LinkOut - more resources
Full Text Sources

