Advances in Alzheimer's disease's pharmacological treatment
- PMID: 36817126
- PMCID: PMC9933512
- DOI: 10.3389/fphar.2023.1101452
Advances in Alzheimer's disease's pharmacological treatment
Abstract
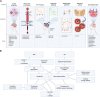
Alzheimer's disease (AD) is the most common type of dementia in the elderly. Several hypotheses emerged from AD pathophysiological mechanisms. However, no neuronal protective or regenerative drug is available nowadays. Researchers still work in drug development and are finding new molecular targets to treat AD. Therefore, this study aimed to summarize main advances in AD pharmacological therapy. Clinical trials registered in the National Library of Medicine database were selected and analyzed accordingly to molecular targets, therapeutic effects, and safety profile. The most common outcome was the lack of efficacy. Only seven trials concluded that tested drugs were safe and induced any kind of therapeutic improvement. Three works showed therapeutic effects followed by toxicity. In addition to aducanumab recent FDA approval, antibodies against amyloid-β (Aβ) showed no noteworthy results. 5-HT6 antagonists, tau inhibitors and nicotinic agonists' data were discouraging. However, anti-Aβ vaccine, BACE inhibitor and anti-neuroinflammation drugs showed promising results.
Keywords: Alzheimer’s disease; drug development; molecular target; new drugs; pharmacological treatment.
Copyright © 2023 Conti Filho, Loss, Marcolongo-Pereira, Rossoni Junior, Barcelos, Chiarelli-Neto, Silva, Passamani Ambrosio, Castro, Teixeira and Mezzomo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alzheimer A. (1906). Über einen eigenartigen schweren Erkrankungsprozeß der Hirnrinde. Neurol. Cent. 23, 1129–1136.
Publication types
LinkOut - more resources
Full Text Sources

