Relationship Between ACSL4-Mediated Ferroptosis and Chronic Obstructive Pulmonary Disease
- PMID: 36817367
- PMCID: PMC9930680
- DOI: 10.2147/COPD.S391129
Relationship Between ACSL4-Mediated Ferroptosis and Chronic Obstructive Pulmonary Disease
Abstract
Purpose: Although cigarette smoke exposure is the major risk factor for chronic obstructive pulmonary disease (COPD), the mechanism is not completely understood. The aim of the present study was to investigate whether ACSL4-mediated ferroptosis in lung epithelial cells plays a part in the COPD development process and its association.
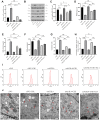
Patients and methods: In this study, animal and cell models of COPD were modelled using cigarette smoke extracts (CSEs), and cell viability, lipid ROS, iron ion deposition, and ferroptosis-related markers were measured in lung tissue and lung epithelial cells following CSE exposure. Morphological changes in mitochondria were observed in lung tissue and epithelial cells of the lung by transmission electron microscope. The expression levels of ACSL4 mRNA and protein in lung tissue and epithelial cells were measured by real-time PCR and Western blotting. In addition, animal-interfering lentivirus and cell-interfering RNA against ACSL4 were constructed in this study, ferroptosis in lung tissue and lung epithelial cells after ACSL4 interference was detected, and ACSL4 mRNA and protein expression levels were detected.
Results: CSE induced ferroptosis in lung tissues and lung epithelial cells, and the expression levels of ACSL4 were elevated in CSE-treated lung tissues and lung epithelial cells. After ACSL4 interference, the expression of ACSL4 decreased, mitochondrial morphology was restored, and ferroptosis in lung tissues and lung epithelial cells was alleviated. Both respiratory frequency and enhanced pause of COPD mice models decreased after ACSL4 interference.
Conclusion: ACSL4-mediated ferroptosis in lung epithelial cells is associated with COPD and positively correlated with ferroptosis in epithelial cells.
Keywords: ACSL4; chronic obstructive pulmonary disease; ferroptosis.
© 2023 Wang and Xia.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

