Identification of Laboratory Biomarkers for Early Detection and Clinical Management of Post-Acute Syndrome Among Survivors of the 2013-2016 West Africa Ebola Outbreak in Sierra Leone
- PMID: 36817368
- PMCID: PMC9930681
- DOI: 10.2147/JBM.S371239
Identification of Laboratory Biomarkers for Early Detection and Clinical Management of Post-Acute Syndrome Among Survivors of the 2013-2016 West Africa Ebola Outbreak in Sierra Leone
Abstract
Background: The clinical management of persistent medical conditions affecting Ebola survivors, generally described as a post-Ebola syndrome, remains a public health concern. We aimed to analyze Ebola survivors' laboratory biomarkers as compared to their non-infected household relatives to identify biomarkers that could guide the identification of survivors at increased risk of developing severe at odds with the non-severe post-Ebola syndrome.
Materials and methods: Data were extracted from medical records of the Ebola survivors clinic, and we included only Ebola survivor's parameters recorded during the first baseline follow-up visit 2 weeks interval after their second negative PCR result. Moreover, household non-infected family contacts of survivors visiting the clinic during the same period were recruited as community control.
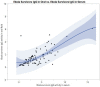
Results: The mean age of survivors was 32.65 (IQR: 15.5, 38.25) years, and Ebola IgG immunoglobulin was detected in all, thus confirming their status. The statistical significance (all p < 0.05) observed in monocyte percentage (MONO%), cluster of differentiation 4 percentage (CD4%), alanine aminotransferase (ALT), creatinine (CREA), and creatinine kinase (C-kinase) proved to be clinically significant as compared to the household relatives' group. Interestingly, the linear regression analysis indicated that the duration at ETU was negatively associated with lymphocyte percentage with a 5% lymphocyte decrease per day spent at ETU. Finally, there was a significant (p < 0.05) association between hematological (Hb, PCV, MCV, MCH), biochemical (ALT, CREA, C-kinase, T-cholesterol, triglycerides) parameters and the risk of developing severe complications.
Conclusion: We recommend clinicians closely monitor Hb, PCV, MCV, MCH, ALT, CREA, C-kinase, T-cholesterol, triglycerides and lymphocytes as clinically relevant laboratory biomarkers to identify survivors at higher risk of developing severe post-acute syndrome upon discharge from Ebola treatment unit including headache, abdominal pain, chest pain, ocular complication, arthralgia, hearing difficulty and erectile dysfunction which can impact health-related quality of life among Ebola survivors.
Keywords: Ebola virus disease; biochemical; biomarkers; hematological; immunological; post-acute syndrome.
© 2023 Guetiya Wadoum et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- World Health Organization (WHO). Ebola data and statistics: situation summary data; 2016. Available from: http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20160511?la.... Accessed February 27, 2022.
-
- Guetiya Wadoum RE, Samin A, Mafopa NG, et al. Mobile health clinic for the medical management of clinical sequelae experienced by survivors of the 2013–2016 Ebola virus disease outbreak in Sierra Leone, West Africa. Eur J Clin Microbiol Infect Dis. 2017;36(11):2193–2200. PMID: 28695354. doi:10.1007/s10096-017-3045-1 - DOI - PubMed
-
- WHO. Clinical care for survivors of Ebola virus disease: interim guidance. Available from: http://apps.who.int/iris/bitstream/10665/204235/1/WHO_EVD_OHE_PED_16.1_e.... Accessed February 26, 2022.
LinkOut - more resources
Full Text Sources
Research Materials

