Persistence of spike-specific immune responses in BNT162b2-vaccinated donors and generation of rapid ex-vivo T cells expansion protocol for adoptive immunotherapy: A pilot study
- PMID: 36817441
- PMCID: PMC9933868
- DOI: 10.3389/fimmu.2023.1061255
Persistence of spike-specific immune responses in BNT162b2-vaccinated donors and generation of rapid ex-vivo T cells expansion protocol for adoptive immunotherapy: A pilot study
Abstract
Introduction: The BNT162b2 mRNA-based vaccine has shown high efficacy in preventing COVID-19 infection but there are limited data on the types and persistence of the humoral and T cell responses to such a vaccine.
Methods: Here, we dissect the vaccine-induced humoral and cellular responses in a cohort of six healthy recipients of two doses of this vaccine.
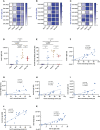
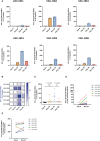
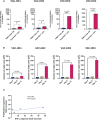
Results and discussion: Overall, there was heterogeneity in the spike-specific humoral and cellular responses among vaccinated individuals. Interestingly, we demonstrated that anti-spike antibody levels detected by a novel simple automated assay (Jess) were strongly correlated (r=0.863, P<0.0001) with neutralizing activity; thus, providing a potential surrogate for neutralizing cell-based assays. The spike-specific T cell response was measured with a newly modified T-spot assay in which the high-homology peptide-sequences cross-reactive with other coronaviruses were removed. This response was induced in 4/6 participants after the first dose, and all six participants after the second dose, and remained detectable in 4/6 participants five months post-vaccination. We have also shown for the first time, that BNT162b2 vaccine enhanced T cell responses also against known human common viruses. In addition, we demonstrated the efficacy of a rapid ex-vivo T cell expansion protocol for spike-specific T cell expansion to be potentially used for adoptive-cell therapy in severe COVID-19, immunocompromised individuals, and other high-risk groups. There was a 9 to 13.7-fold increase in the number of expanded T cells with a significant increase of anti-spike specific response showing higher frequencies of both activation and cytotoxic markers. Interestingly, effector memory T cells were dominant in all four participants' CD8+ expanded memory T cells; CD4+ T cells were dominated by effector memory in 2/4 participants and by central memory in the remaining two participants. Moreover, we found that high frequencies of CD4+ terminally differentiated memory T cells were associated with a greater reduction of spike-specific activated CD4+ T cells. Finally, we showed that participants who had a CD4+ central memory T cell dominance expressed a high CD69 activation marker in the CD4+ activated T cells.
Keywords: COVID-19 vaccine; SARS-CoV-2; spike-specific T cells expansion; spike-specific immune responses; surrogate neutralization.
Copyright © 2023 Mestiri, Merhi, Inchakalody, Taib, Smatti, Ahmad, Raza, Ali, Hydrose, Fernandes, Ansari, Sahir, Al-Zaidan, Jalis, Ghoul, Allahverdi, Al Homsi, Uddin, Jeremijenko, Nimir, Abu-Raddad, Abid, Zaqout, Alfheid, Saqr, Omrani, Hssain, Al Maslamani, Yassine and Dermime.
Conflict of interest statement
SD, SM, MM, VPI, NT, FA, AR, SH, QF, AWA, FS, LA, MJ, MG, NA, MUA, SU, AMJ, MN, ASO, AAH, MAM, FBA, AZ, SRA, and HMHS were employed by Hamad Medical Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

