The role of glutamate receptors in the regulation of the tumor microenvironment
- PMID: 36817470
- PMCID: PMC9929049
- DOI: 10.3389/fimmu.2023.1123841
The role of glutamate receptors in the regulation of the tumor microenvironment
Abstract
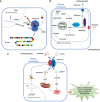
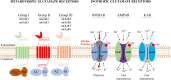
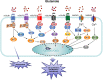
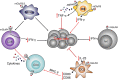
Glutamate, as one of the most important carbon sources in the TCA cycle, is central in metabolic processes that will subsequently influence tumor progression. Several factors can affect the expression of glutamate receptors, playing either a tumor-promoting or tumor-suppressor role in cancer. Thus, the activation of glutamate receptors by the ligand could play a role in tumor development as ample studies have demonstrated the expression of glutamate receptors in a broad range of tumor cells. Glutamate and its receptors are involved in the regulation of different immune cells' development and function, as suggested by the receptor expression in immune cells. The activation of glutamate receptors can enhance the effectiveness of the effector's T cells, or decrease the cytokine production in immunosuppressive myeloid-derived suppressor cells, increasing the antitumor immune response. These receptors are essential for the interaction between tumor and immune cells within the tumor microenvironment (TME) and the regulation of antitumor immune responses. Although the role of glutamate in the TCA cycle has been well studied, few studies have deeply investigated the role of glutamate receptors in the regulation of cancer and immune cells within the TME. Here, by a systematic review of the available data, we will critically assess the physiopathological relevance of glutamate receptors in the regulation of cancer and immune cells in the TME and provide some unifying hypotheses for futures research on the role of glutamate receptors in the immune modulation of the tumor.
Keywords: glutamate; glutamate receptors; immunoregulation; metabolic processes; tumor micro-environment.
Copyright © 2023 Koda, Hu, Ju, Sun, Shao, Tang, Zheng and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
-
Selenium for preventing cancer.Cochrane Database Syst Rev. 2018 Jan 29;1(1):CD005195. doi: 10.1002/14651858.CD005195.pub4. Cochrane Database Syst Rev. 2018. PMID: 29376219 Free PMC article.
Cited by
-
Glutamate promotes CCL2 expression to recruit tumor-associated macrophages by restraining EZH2-mediated histone methylation in hepatocellular carcinoma.Oncoimmunology. 2025 Dec;14(1):2497172. doi: 10.1080/2162402X.2025.2497172. Epub 2025 Apr 24. Oncoimmunology. 2025. PMID: 40271976 Free PMC article.
-
Glutamate transporter SLC1A6 promotes resistance to immunotherapy in cancer.Cancer Immunol Immunother. 2025 Jun 7;74(8):240. doi: 10.1007/s00262-025-04074-4. Cancer Immunol Immunother. 2025. PMID: 40481876 Free PMC article.
-
Proteome characterization of extracellular vesicles from human milk: Uncovering the surfaceome by a lipid-based protein immobilization technology.J Extracell Biol. 2024 Nov 7;3(11):e70020. doi: 10.1002/jex2.70020. eCollection 2024 Nov. J Extracell Biol. 2024. PMID: 39512873 Free PMC article.
-
Expression of mGluR5 in Pediatric Hodgkin and Non-Hodgkin lymphoma-A Comparative Analysis of Immunohistochemical and Clinical Findings Regarding the Association between Tumor and Paraneoplastic Neurological Disease.Cancers (Basel). 2024 Jul 4;16(13):2452. doi: 10.3390/cancers16132452. Cancers (Basel). 2024. PMID: 39001514 Free PMC article.
-
GRIK1 promotes glioblastoma malignancy and is a novel prognostic factor of poor prognosis.Oncol Res. 2024 Mar 20;32(4):727-736. doi: 10.32604/or.2023.043391. eCollection 2024. Oncol Res. 2024. PMID: 38560566 Free PMC article.
References
-
- Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, et al. . Mitochondrial localization and structure-based phosphate activation mechanism of glutaminase c with implications for cancer metabolism. Proc Natl Acad Sci U S A (2012) 109(4):1092–7. doi: 10.1073/pnas.1112495109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

