Progress on diagnosis and treatment of drug-resistant tuberculosis in line with World Health Organization recommendations in six priority countries in the Western Pacific Region
- PMID: 36817504
- PMCID: PMC9912284
- DOI: 10.5365/wpsar.2022.13.4.972
Progress on diagnosis and treatment of drug-resistant tuberculosis in line with World Health Organization recommendations in six priority countries in the Western Pacific Region
Abstract
Background: Diagnosis and treatment of drug-resistant tuberculosis (DR-TB) have radically changed in accordance with recommendations from the World Health Organization (WHO) in the past decade, allowing rapid and simple diagnosis and shorter treatment duration with new and repurposed drugs.
Methods: A descriptive analysis of the status and progress of DR-TB diagnosis and treatment in six priority countries in the Western Pacific Region was conducted using information from interviews with countries and the WHO TB database.
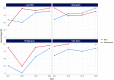
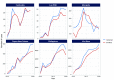
Results: Over the past decade, the use of Xpert MTB/RIF has increased in the six priority countries, in parallel with implementation of national policies and algorithms to use Xpert MTB/RIF as an initial diagnostic test for TB and detection of rifampicin resistance. This has resulted in increases in the number of people diagnosed with multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB). Shorter treatment regimens with new and repurposed drugs have also been adopted for MDR/RR-TB cases, alongside a decentralized model of care, leading to improved treatment outcomes.
Discussion: The Western Pacific Region has achieved considerable progress in the diagnosis and treatment of DR-TB, in line with the evolving WHO recommendations in the past decade. The continued commitment of Member States is needed to address remaining challenges, such as the impact of the coronavirus disease pandemic, suboptimal management and health system issues.
(c) 2022 The authors; licensee World Health Organization.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/346387, accessed 19 July 2022.
-
- Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M,et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;S2213–2600(17)30079–6. doi:10.1016/S2213-2600(17)30079-6 pmid:28344011.10.1016/S2213-2600(17)30079-6 - DOI - DOI - PubMed
-
- Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva: World Health Organization; 2013. Available from: https://apps.who.int/iris/handle/10665/112472, accessed 19 July 2022. - PubMed
-
- The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs: policy guidance. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/246131, accessed 19 July 2022.
-
- WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/250125, acessed 19 July 2022. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
