Integration of parallel metabolomics and transcriptomics reveals metabolic patterns in porcine oocytes during maturation
- PMID: 36817597
- PMCID: PMC9929430
- DOI: 10.3389/fendo.2023.1131256
Integration of parallel metabolomics and transcriptomics reveals metabolic patterns in porcine oocytes during maturation
Abstract
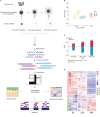
Well-controlled metabolism is the prerequisite for optimal oocyte development. To date, numerous studies have focused mainly on the utilization of exogenous substrates by oocytes, whereas the underlying mechanism of intrinsic regulation during meiotic maturation is less characterized. Herein, we performed an integrated analysis of parallel metabolomics and transcriptomics by isolating porcine oocytes at three time points, cooperatively depicting the global picture of the metabolic patterns during maturation. In particular, we identified the novel metabolic features during porcine oocyte meiosis, such as the fall in bile acids, the active one-carbon metabolism and a progressive decline in nucleotide metabolism. Collectively, the current study not only provides a comprehensive multiple omics data resource, but also may facilitate the discovery of molecular biomarkers that could be used to predict and improve oocyte quality.
Keywords: energy metabolism; metabolomics; oocyte; reproduction; transcriptomics.
Copyright © 2023 Gao, Chen, Chen, Zhu, Wang, Yang, Wang, Wang and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

