High-Frequency 3D Photoacoustic Computed Tomography Using an Optical Microring Resonator
- PMID: 36818003
- PMCID: PMC9933894
- DOI: 10.34133/2022/9891510
High-Frequency 3D Photoacoustic Computed Tomography Using an Optical Microring Resonator
Abstract
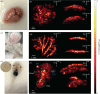
3D photoacoustic computed tomography (3D-PACT) has made great advances in volumetric imaging of biological tissues, with high spatial-temporal resolutions and large penetration depth. The development of 3D-PACT requires high-performance acoustic sensors with a small size, large detection bandwidth, and high sensitivity. In this work, we present a new high-frequency 3D-PACT system that uses a micro-ring resonator (MRR) as the acoustic sensor. The MRR sensor has a size of 80 μm in diameter, and was fabricated using the nanoimprint lithography technology. Using the MRR sensor, we have developed a transmission-mode 3D-PACT system that has achieved a detection bandwidth of ~23 MHz, an imaging depth of ~8 mm, a lateral resolution of 114 μm, and an axial resolution of 57 μm. We have demonstrated the 3D PACT's performance on in vitro phantoms, ex vivo mouse brain, and in vivo mouse ear and tadpole. The MRR-based 3D-PACT system can be a promising tool for structural, functional, and molecular imaging of biological tissues at depths.
Keywords: All-optical system; High frequency detection; Micro-ring resonator; Photoacoustic computed tomography; Three-dimensional imaging; Transmission-mode imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Taruttis A., and Ntziachristos V., “Advances in real-time multispectral optoacoustic imaging and its applications,” Nature Photonics, vol. 9, no. 4, pp. 219–227, 2015
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

