Characterization of human induced pluripotent stems cells: Current approaches, challenges, and future solutions
- PMID: 36818379
- PMCID: PMC9929203
- DOI: 10.1016/j.btre.2023.e00784
Characterization of human induced pluripotent stems cells: Current approaches, challenges, and future solutions
Abstract
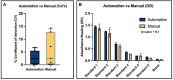
Human induced pluripotent stem cells (iPSC) have demonstrated massive potentials for use in regenerative and personalized medicine due to their ability to expand in culture and differentiate into specialized cells with therapeutic benefits. However, in order to industrialize iPSC-derived therapies, it is necessary to address the existing challenges surrounding the analytics implemented in the manufacturing process to evaluate and monitor cell expansion, differentiation, and quality of the final products. Here, we review some of the key analytical methods used as part of identity, potency, or safety for in-process or final product release testing and highlighted the challenges and potential solutions for consideration in the Chemistry, Manufacturing and Controls (CMC) strategy for iPSC-based therapies. Some of the challenges associated with characterization and testing of iPSC-based products are related to the choice of analytical technology (to ensure fit-for-purpose), assay reliability and robustness. Automation of analytical methods may be required to reduce hands on time, and improve reliability of the methods through reducing assay variability. Indeed, we have shown that automation of analytical methods is feasible (evaluated using an ELISA based assay) and would result in more precise measurements (demonstrated by lower co-efficient of Variation and standard deviation), less hands-on time, and swift compared to a manually run assay. Therefore, in order to support commercialization of iPSC-based therapies we suggest a well-designed testing strategy to be established in the development phase while incorporating robust, reproducible, reliable, and potentially automated analytics in the manufacturing process.
Keywords: Analytical methods; Assay robustness; Automation; Cell therapies; iPSCs.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that there are no competing interests. The authors (Sahana Suresh Babu, Haritha Duvvuru, Jillian Baker, Stephanie Switalski, Mehdi Shafa, Krishna Morgan Panchalingam, Saedeh Dadgar, Justin Beller and Behnam Ahmadian Baghbaderani) are full time employees of Lonza. The results described do not describe or endorse any commercial product.
Figures


References
-
- Shafa M., Walsh T., Panchalingam K.M., Richardson T., Menendez L., Tian X., Suresh Babu S., Dadgar S., Beller J., Yang F., Baghbaderani B.A. Long-Term stability and differentiation potential of cryopreserved cGMP-compliant human induced pluripotent stem cells. Int. J. Mol. Sci. 2019;21(1) - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
