Mechanical Thrombectomy for Acute and Subacute Blocked Arteries and Veins in the Lower Limbs: A Health Technology Assessment
- PMID: 36818453
- PMCID: PMC9899119
Mechanical Thrombectomy for Acute and Subacute Blocked Arteries and Veins in the Lower Limbs: A Health Technology Assessment
Abstract
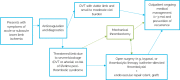
Background: A blockage to the blood vessels in the lower extremities may cause pain and discomfort. If left unmanaged, it may lead to amputation or chronic disability, such as in the form of post-thrombotic syndrome. We conducted a health technology assessment of mechanical thrombectomy (MT) devices, which are proposed to remove a blood clot, which may form in the arteries or veins of the lower legs. This evaluation considered blockages in the veins and arteries separately, and included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding MT for lower limb blockages, patient preferences and values, and clinical and health system stakeholders' perspectives.
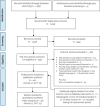
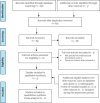
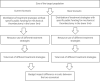
Method: We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Cochrane tool for randomized controlled trials or the risk of bias among non-randomized studies (RoBANS) tool for nonrandomized studies, and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search. We did not conduct a primary economic evaluation since the clinical evidence is highly uncertain. We also analyzed the budget impact of publicly funding MT treatment for inpatients with arterial acute limb ischemia and acute deep vein thrombosis (DVT) in the lower limb in Ontario. To contextualize the potential value of MT, we spoke with people with acute DVT. To understand the barriers and facilitators of accessing MT, we surveyed clinical and health system stakeholders to gain their perspectives.
Results: We included 40 studies (3 randomized controlled trials and 37 observational studies) in the clinical evidence review. For patients who experience arterial acute limb ischemia, compared with catheter-directed thrombolysis (CDT) alone, MT has greater technical success and patency and reduced hospital length of stay, but the evidence for these outcomes is uncertain (GRADE: Very low). Mechanical thrombectomy may reduce the volume of thrombolytic medication required and CDT infusion time (a determinant for intensive care unit [ICU] need) in patients experiencing acute DVT, but it is uncertain if this is to a meaningful degree (GRADE: Moderate to Very low). It may also reduce the proportion of people who experience post-thrombotic syndrome and overall hospital length of stay, but it is uncertain (GRADE: Very low).We estimated that publicly funding MT for people with arterial acute limb ischemia in Ontario would lead to an annual cost savings of $0.17 million in year 1 to $0.14 million in year 5, for a total savings of $0.83 million over 5 years. This cost savings was mainly attributed to reduced ICU stays among people who received MT, but the results had considerable uncertainty. For the population with acute DVT, publicly funding MT would lead to an additional cost of $0.77 million in year 1 to $1.44 million in year 5, for a total additional cost of $5.5 million over 5 years.The people with acute DVT with whom we spoke reported that MT was generally seen as a positive option, and those who had undergone the procedure reported positively on its value as a treatment to quickly remove a clot. Accessing treatment for DVT could be a barrier, especially in more remote areas of Ontario.Clinicians using the technology advised that facilitators to accessing the technology included perceived improvements in patient outcomes, resourcing requirements, addressing unmet needs, and avoidance of ICU stay. The main barrier identified was cost. Clinicians who were not using the technology advised that barriers were low case-use volume, along with costs for the equipment and for health human resources.
Conclusions: Mechanical thrombectomy may have greater technical success and patency and reduce hospital length of stay for patients experiencing an arterial acute limb ischemia and, for patients with an acute DVT, it may reduce CDT volume and infusion time, the proportion of people who experience post-thrombotic syndrome, and hospital length of stay. Mechanical thrombectomy may reduce the associated ICU costs, but it has higher equipment costs compared with usual care. Publicly funding MT in Ontario for populations with arterial acute limb ischemia may not lead to a substantial budget increase to the province. Publicly funding MT for acute DVT would lead to an additional cost of $5.5 million over 5 years. For people with acute DVT, MT was seen as a potential positive treatment option to remove the clot quickly. Overall, the majority of clinical stakeholders we engaged with (including both those with and without experience with MT) were supportive of the use of the technology.
Copyright © King's Printer for Ontario, 2023.
Figures






















References
-
- Engelberger RP, Spirk D, Willenberg T, Alatri A, Do DD, Baumgartner I, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(1). - PubMed
-
- Healthwise Staff. Venous thrombus and embolus [Internet]. Alberta: MyHealth.Alberta.ca; 2019. [June 22, 2021]. Available from: https://myhealth.alberta.ca/health/Pages/conditions.aspx?hwid=tp12576&la...
-
- Patel NH, Krishnamurthy VN, Kim S, Saad WE, Ganguli S, Walker TG, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
