Influence of the electrocatalyst layer thickness on alkaline DEFC performance
- PMID: 36818600
- PMCID: PMC9926948
- DOI: 10.1039/d2se01729f
Influence of the electrocatalyst layer thickness on alkaline DEFC performance
Abstract
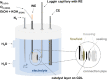
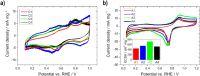
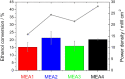
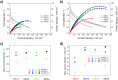
Determining the optimum layer thickness, for the anode and cathode, is of utmost importance for minimizing the costs of the alkaline direct ethanol fuel cell (DEFC) without lowering the electrochemical performance. In this study, the influence of layer thickness on the performance of the ethanol oxidation reaction (EOR) and oxygen reduction reaction (ORR) in an alkaline medium and resistance was investigated. The prepared gas diffusion electrodes (GDEs) were fully characterized, with scanning electron microscopy to determine the layer thickness and electrochemically in half-cell configuration. Cyclic voltammetry and polarization curve measurements were used to determine the oxidation and reduction processes of the metals, the electrochemical active surface area, and the activity towards the ORR and EOR. It was demonstrated that realistic reaction conditions can be achieved with simple and fast half-cell GDE measurements. Single cell measurements were conducted to evaluate the influence of factors, such as membrane or ethanol crossover. In addition, electrochemical impedance spectra investigation was performed to identify the effect of layer thickness on resistance. This successfully demonstrated that the optimal layer thicknesses and high maximum power density values (120 mW cm-2) were achieved with the Pt-free catalysts and membranes used.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Yu E. H. Wang X. Krewer U. Li L. Scott K. Energy Environ. Sci. 2012;5:5668–5680. doi: 10.1039/C2EE02552C. - DOI
-
- Zhao T. S. Li Y. S. Shen S. Y. Front. Energy Power Eng. China. 2010;4:443–458. doi: 10.1007/s11708-010-0127-5. - DOI
-
- Monyoncho E. A. Woo T. K. Baranova E. A. SPR Electrochem. 2019;15:1–57.
-
- Kamarudin M. Z. F. Kamarudin S. K. Masdar M. S. Daud W. R. W. Int. J. Hydrogen Energy. 2013;38:9438–9453. doi: 10.1016/j.ijhydene.2012.07.059. - DOI
-
- Badwal S. P. S. Giddey S. Kulkarni A. Goel J. Basu S. Appl. Energy. 2015;145:80–103. doi: 10.1016/j.apenergy.2015.02.002. - DOI
LinkOut - more resources
Full Text Sources
