Targeting BRAF V600E in metastatic colorectal cancer: where are we today?
- PMID: 36819812
- PMCID: PMC9934973
- DOI: 10.3332/ecancer.2022.1489
Targeting BRAF V600E in metastatic colorectal cancer: where are we today?
Abstract
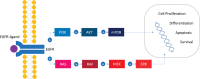
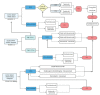
Colorectal cancer (CRC) is the second most frequent cause of direct cancer death worldwide. The study of the molecular state of oncogenes has predictive and prognostic value in metastatic CRC (mCRC). The B-raf proto-oncogene (BRAF) gene mutation represents the 8%-12% of all mutations in mCRC. The BRAF V600E mutation, considered the most common alteration of BRAF, corresponds to a constitutive kinase with a high activating capacity of the RAS/RAF/MEK/ERK pathway after a cascade of successive phosphorylations in the transcription of genes. BRAF V600E mutation is more prevalent in women, elderly, right-sided colon cancer and Caucasian population. Unfortunately, it is considered a poor predictive and prognosis biomarker. Patients with mCRC BRAF V600E mutated (BRAFm) are generally associated with poor response to chemotherapy and short progression-free survival and overall survival. Recently, randomised clinical trials have studied the combination of different chemotherapy regimens with angiogenic inhibitors in mCRC BRAFm. In addition, new anti-BRAF and immunotherapy agents have also been studied in this population, with positive results. The objective of this review is to acknowledge the biology and molecular pathway of BRAF, critically analyse the clinical trials and the therapy options published until today and evaluate the options of treatment according to the patient's clinical presentation.
Keywords: antineoplastic agents; colorectal neoplasms; drug therapy; immunotherapy.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
The authors declare that they have no financial or non-financial conflicts of interest.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–249. - PubMed
-
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wildtype metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. - DOI - PubMed
-
- Rivera F, Karthaus M, Hecht JR, et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during firstline treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis. 2017;32:1179–1190. doi: 10.1007/s00384-017-2800-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
