Transcutaneous Spinal Stimulation From Adults to Children: A Review
- PMID: 36819932
- PMCID: PMC9936896
- DOI: 10.46292/sci21-00084
Transcutaneous Spinal Stimulation From Adults to Children: A Review
Abstract
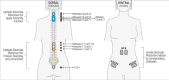
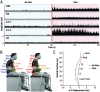
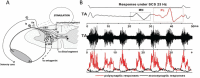
Neuromodulation via spinal stimulation is a promising therapy that can augment the neuromuscular capacity for voluntary movements, standing, stepping, and posture in individuals with spinal cord injury (SCI). The spinal locomotor-related neuronal network known as a central pattern generator (CPG) can generate a stepping-like motor output in the absence of movement-related afferent signals from the limbs. Using epidural stimulation (EP) in conjunction with activity-based locomotor training (ABLT), the neural circuits can be neuromodulated to facilitate the recovery of locomotor functions in persons with SCI. Recently, transcutaneous spinal stimulation (scTS) has been developed as a noninvasive alternative to EP. Early studies of scTS at thoracolumbar, coccygeal, and cervical regions have demonstrated its effectiveness in producing voluntary leg movements, posture control, and independent standing and improving upper extremity function in adults with chronic SCI. In pediatric studies, the technology of spinal neuromodulation is not yet widespread. There are a limited number of publications reporting on the use of scTS in children and adolescents with either cerebral palsy, spina bifida, or SCI.
Keywords: motor recovery; neuromodulation; pediatric; spinal cord injury; spinal stimulation; transcutaneous spinal cord stimulation.
©2023 American Spinal Injury Association.
Conflict of interest statement
Conflicts of Interest Dr. Gerasimenko has a relationship with Cosyma, Ltd, Moscow Russia. He is a founder and Scientific Director of Cosyma and holds a patent on a stimulator used in research. He is funded by National Institutes of Health grant (R01 NS102920-01A1), the Craig H. Neilsen Foundation, and the Kentucky Spinal Cord and Head Injury Research Trust Fund. Dr. Behrman has received support from the Jewish Heritage Foundation for Excellence, National Center for Neuromodulation for Rehabilitation, Kentucky Spinal Cord and Head Injury Research Board, The Craig H. Neilsen Foundation, and Oxford University Press. Kosair Charities made payments to University of Louisville Foundation Inc., and Kosair Charities made payment directly to the institution from 2014-2016. Drs. Lucas and Singh received support from Kosair Charities (OGMB141540), National Center of Neuromodulation for Rehabilitation (NM4R) (CCDN190765), Kentucky Spinal Cord and Head Injury Research Board (OGMB210076), and the Jewish Heritage Foundation for Excellence (OGMN190574A). Dr. Martin received support from Orokawa Foundation, Niche Biomedical, and Kennedy Krieger Institute and research support to the Kennedy Krieger Institute.
Figures


Similar articles
-
Safety and Feasibility of Cervical and Thoracic Transcutaneous Spinal Cord Stimulation to Improve Hand Motor Function in Children With Chronic Spinal Cord Injury.Neuromodulation. 2024 Jun;27(4):661-671. doi: 10.1016/j.neurom.2023.04.475. Epub 2023 Jun 1. Neuromodulation. 2024. PMID: 37269282
-
Transcutaneous electrical spinal-cord stimulation in humans.Ann Phys Rehabil Med. 2015 Sep;58(4):225-231. doi: 10.1016/j.rehab.2015.05.003. Epub 2015 Jul 20. Ann Phys Rehabil Med. 2015. PMID: 26205686 Free PMC article.
-
Priming locomotor training with transspinal stimulation in people with spinal cord injury: study protocol of a randomized clinical trial.Trials. 2023 Feb 25;24(1):145. doi: 10.1186/s13063-023-07193-4. Trials. 2023. PMID: 36841773 Free PMC article.
-
The physiological basis of neurorehabilitation--locomotor training after spinal cord injury.J Neuroeng Rehabil. 2013 Jan 21;10:5. doi: 10.1186/1743-0003-10-5. J Neuroeng Rehabil. 2013. PMID: 23336934 Free PMC article. Review.
-
Emergence of Epidural Electrical Stimulation to Facilitate Sensorimotor Network Functionality After Spinal Cord Injury.Neuromodulation. 2019 Apr;22(3):244-252. doi: 10.1111/ner.12938. Epub 2019 Mar 6. Neuromodulation. 2019. PMID: 30840354 Review.
Cited by
-
Restoration of Over-Ground Walking via Non-Invasive Neuromodulation Therapy: A Single-Case Study.J Clin Med. 2023 Nov 28;12(23):7362. doi: 10.3390/jcm12237362. J Clin Med. 2023. PMID: 38068414 Free PMC article.
-
Activity-Based Restorative Therapy Promotes Progression from Asymmetry to Symmetry in Posture and Gait in a Child with Chronic, Incomplete Spinal Cord Injury.Children (Basel). 2023 Mar 20;10(3):594. doi: 10.3390/children10030594. Children (Basel). 2023. PMID: 36980152 Free PMC article.
-
Synergistic effects of transcutaneous spinal stimulation and neuromuscular electrical stimulation on lower limb force production: Time to deliver.PLoS One. 2024 Aug 30;19(8):e0296613. doi: 10.1371/journal.pone.0296613. eCollection 2024. PLoS One. 2024. PMID: 39213293 Free PMC article.
-
Combining transcutaneous spinal stimulation and functional electrical stimulation increases force generated by lower limbs: When more is more.bioRxiv [Preprint]. 2023 Dec 23:2023.12.22.573119. doi: 10.1101/2023.12.22.573119. bioRxiv. 2023. PMID: 38187778 Free PMC article. Preprint.
-
Different Factors Influencing Postural Stability during Transcutaneous Electrical Stimulation of the Cervical Spinal Cord.J Funct Morphol Kinesiol. 2024 Aug 22;9(3):142. doi: 10.3390/jfmk9030142. J Funct Morphol Kinesiol. 2024. PMID: 39311250 Free PMC article.
References
-
- Gerasimenko Y, Daniel O, Regnaux J, Combeaud M, Bussel B. Mechanisms of locomotor activity generation under epidural spinal cord stimulation. Dengler R, Kossev A, editors. Sensorimotor Control NATO Science Series 1 Life and Behavioural Sciences . 2001;326:164–171. In. eds.
-
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann NY Acad Sc . 1998;860:360–376. - PubMed
-
- Richardson RR, McLone DG. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol . 1978;9(3):153–155. - PubMed
-
- Siegfried J. Sensory thalamic neurostimulation for chronic pain. Pacing Clin Electrophysiol . 1987;10(1 Pt 2):209–212. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
