Multifunctional Nano-Realgar Hydrogel for Enhanced Glioblastoma Synergistic Chemotherapy and Radiotherapy: A New Paradigm of an Old Drug
- PMID: 36820060
- PMCID: PMC9938708
- DOI: 10.2147/IJN.S394377
Multifunctional Nano-Realgar Hydrogel for Enhanced Glioblastoma Synergistic Chemotherapy and Radiotherapy: A New Paradigm of an Old Drug
Abstract
Purpose: Realgar, as a kind of traditional mineral Chinese medicine, can inhibit multiple solid tumor growth and serve as an adjuvant drug in cancer therapy. However, the extremely low solubility and poor body absorptive capacity limit its application in clinical medicine. To overcome this therapeutic hurdle, realgar can here be fabricated into a nano-realgar hydrogel with enhanced chemotherapy and radiotherapy (RT) ability. Our objective is to evaluate the superior biocompatibility and anti-tumor activity of nano-realgar hydrogel.
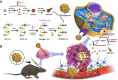
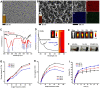
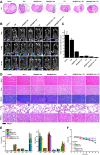
Methods: We have successfully synthesized nano-realgar quantum dots (QDs) coupling with 6-AN molecules (NRA QDs) and further encapsulated with a pH-sensitive dextran hydrogel carrier with hyaluronic acid coating (DEX-HA gel) to promote bioavailability, eventually forming a multifunctional nano-realgar hydrogel (NRA@DH Gel). To better investigate the tumor therapy efficiency of the NRA@DH Gel, we have established the mice in situ bearing GL261 brain glioblastoma as animal models assigned to receive intratumor injection of NRA@DH Gel.
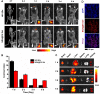
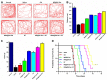
Results: The designed NRA@DH Gel as an antitumor drug can not only exert the prominent chemotherapy effect but also as a "sustainable reactive oxygen species (ROS) generator" can inhibit in the pentose phosphate pathway (PPP) metabolism and reduce the production of nicotinamide adenine dinucleotide phosphate (NADPH), thereby inhibiting the conversion of glutathione disulfide (GSSG) to glutathione (GSH), reducing GSH concentrations in tumor cells, triggering the accumulation of ROS, and finally enhancing the effectiveness of RT.
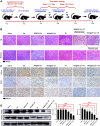
Conclusion: Through the synergistic effect of chemotherapy and RT, NRA@DH Gel effectively inhibited the proliferation and migration of tumor cells, suppressed tumor growth, improved motor coordination, and prolonged survival in tumor-bearing mice. Our work aims to improve the NRA@DH Gel-mediated synergistic chemotherapy and RT will endow a "promising future" for the old drug in clinically comprehensive applications.
Keywords: NRA@DH Gel; ROS; glioma; inhibition of the PPP; radiosensitization; synergistic therapy.
© 2023 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, Phase 3 study. Lancet Oncology. 2021;22(6):813–823. doi:10.1016/s1470-2045(21)00090-5 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

