Protection against severe COVID-19 after second booster dose of adapted bivalent (original/Omicron BA.4-5) mRNA vaccine in persons ≥ 60 years, by time since infection, Italy, 12 September to 11 December 2022
- PMID: 36820640
- PMCID: PMC9951255
- DOI: 10.2807/1560-7917.ES.2023.28.8.2300105
Protection against severe COVID-19 after second booster dose of adapted bivalent (original/Omicron BA.4-5) mRNA vaccine in persons ≥ 60 years, by time since infection, Italy, 12 September to 11 December 2022
Abstract
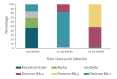
Effectiveness against severe COVID-19 of a second booster dose of the bivalent (original/BA.4-5) mRNA vaccine 7-90 days post-administration, relative to a first booster dose of an mRNA vaccine received ≥ 120 days earlier, was ca 60% both in persons ≥ 60 years never infected and in those infected > 6 months before. Relative effectiveness in those infected 4-6 months earlier indicated no significant additional protection (10%; 95% CI: -44 to 44). A second booster vaccination 6 months after the latest infection may be warranted.
Keywords: COVID-19; Italy; SARS-CoV-2; bivalent mRNA vaccines; effectiveness; elderly population.
Figures
References
-
- Italian National Institute of Health (ISS). EpiCentro. Monitoraggio delle varianti del virus SARS-CoV-2 di interesse in sanità pubblica in Italia – Indagini rapide. [Monitoring of the SARS-CoV-2 variants of interest for public health in Italy – rapid investigations]. Italian. Rome: ISS. [Accessed: 1 Feb 2023]. Available from: https://www.epicentro.iss.it/coronavirus/sars-cov-2-monitoraggio-variant...
-
- Italian Government, Presidency of the Council of Ministers. Report vaccini anti COVID19. [COVID-19 vaccination report]. Rome: Italian Government; 2020. Italian. Available from: https://www.governo.it/it/dipartimenti/commissario-straordinario-lemerge...
-
- Italian National Institute of Health (ISS). COVID-19 integrated surveillance: key national data. Rome: ISS. [Accessed: 1 Feb 2023]. Available from: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-survei...
-
- European Centre for Disease Prevention and Control (ECDC). Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. Stockholm: ECDC. [Accessed: 1 Feb 2023]. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition
-
- World Health Organization (WHO). International guidelines for certification and classification (coding) of COVID-19 as cause of death. Geneva: WHO; 2020. Available from: https://www.who.int/publications/m/item/international-guidelines-for-cer...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
