Probenecid affects muscle Ca2+ homeostasis and contraction independently from pannexin channel block
- PMID: 36820799
- PMCID: PMC9998970
- DOI: 10.1085/jgp.202213203
Probenecid affects muscle Ca2+ homeostasis and contraction independently from pannexin channel block
Abstract
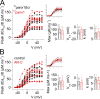
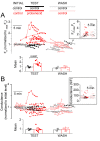
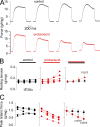
Tight control of skeletal muscle contractile activation is secured by the excitation-contraction (EC) coupling protein complex, a molecular machinery allowing the plasma membrane voltage to control the activity of the ryanodine receptor Ca2+ release channel in the sarcoplasmic reticulum (SR) membrane. This machinery has been shown to be intimately linked to the plasma membrane protein pannexin-1 (Panx1). We investigated whether the prescription drug probenecid, a widely used Panx1 blocker, affects Ca2+ signaling, EC coupling, and muscle force. The effect of probenecid was tested on membrane current, resting Ca2+, and SR Ca2+ release in isolated mouse muscle fibers, using a combination of whole-cell voltage-clamp and Ca2+ imaging, and on electrically triggered contraction of isolated muscles. Probenecid (1 mM) induces SR Ca2+ leak at rest and reduces peak voltage-activated SR Ca2+ release and contractile force by 40%. Carbenoxolone, another Panx1 blocker, also reduces Ca2+ release, but neither a Panx1 channel inhibitory peptide nor a purinergic antagonist affected Ca2+ release, suggesting that probenecid and carbenoxolone do not act through inhibition of Panx1-mediated ATP release and consequently altered purinergic signaling. Probenecid may act by altering Panx1 interaction with the EC coupling machinery, yet the implication of another molecular target cannot be excluded. Since probenecid has been used both in the clinic and as a masking agent for doping in sports, these results should encourage evaluation of possible effects on muscle function in treated individuals. In addition, they also raise the question of whether probenecid-induced altered Ca2+ homeostasis may be shared by other tissues.
© 2023 Jaque-Fernandez et al.
Figures








References
-
- Aguilar-Perez, A., Pacheco-Costa R., Atkinson E.G., Deosthale P., Davis H.M., Essex A.L., Dilley J.E., Gomez L., Rupert J.E., Zimmers T.A., et al. . 2019. Age- and sex-dependent role of osteocytic pannexin1 on bone and muscle mass and strength. Sci. Rep. 9:13903. 10.1038/s41598-019-50444-1 - DOI - PMC - PubMed
-
- Arias-Calderón, M., Almarza G., Díaz-Vegas A., Contreras-Ferrat A., Valladares D., Casas M., Toledo H., Jaimovich E., and Buvinic S.. 2016. Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle. Skelet. Muscle. 6:15. 10.1186/s13395-016-0087-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

