A Brief Introduction to Chemical Reaction Optimization
- PMID: 36820880
- PMCID: PMC10037254
- DOI: 10.1021/acs.chemrev.2c00798
A Brief Introduction to Chemical Reaction Optimization
Abstract
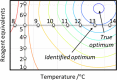
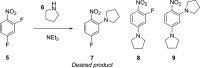
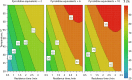
From the start of a synthetic chemist's training, experiments are conducted based on recipes from textbooks and manuscripts that achieve clean reaction outcomes, allowing the scientist to develop practical skills and some chemical intuition. This procedure is often kept long into a researcher's career, as new recipes are developed based on similar reaction protocols, and intuition-guided deviations are conducted through learning from failed experiments. However, when attempting to understand chemical systems of interest, it has been shown that model-based, algorithm-based, and miniaturized high-throughput techniques outperform human chemical intuition and achieve reaction optimization in a much more time- and material-efficient manner; this is covered in detail in this paper. As many synthetic chemists are not exposed to these techniques in undergraduate teaching, this leads to a disproportionate number of scientists that wish to optimize their reactions but are unable to use these methodologies or are simply unaware of their existence. This review highlights the basics, and the cutting-edge, of modern chemical reaction optimization as well as its relation to process scale-up and can thereby serve as a reference for inspired scientists for each of these techniques, detailing several of their respective applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
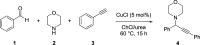
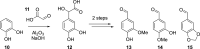

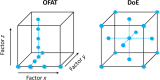
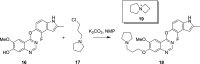




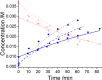






References
-
- Taylor C. J.; Baker A.; Chapman M. R.; Reynolds W. R.; Jolley K. E.; Clemens G.; Smith G. E.; Blacker A. J.; Chamberlain T. W.; Christie S. D.; et al. Flow Chemistry for Process Optimisation Using Design of Experiments. J. Flow. Chem. 2021, 11, 75–86. 10.1007/s41981-020-00135-0. - DOI
-
- Tsay C.; Pattison R. C.; Piana M. R.; Baldea M. A Survey of Optimal Process Design Capabilities and Practices in the Chemical and Petrochemical Industries. Comput. Chem. Eng. 2018, 112, 180–189. 10.1016/j.compchemeng.2018.01.012. - DOI
-
- Andraos J.Designing a Green Organic Chemistry Lecture Course. In Green Organic Chemistry in Lecture and Laboratory, 1st ed.; CRC Press: Boca Raton, FL, 2012; pp 29–68.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

