First detected geographical cluster of BoDV-1 encephalitis from same small village in two children: therapeutic considerations and epidemiological implications
- PMID: 36821024
- PMCID: PMC9947883
- DOI: 10.1007/s15010-023-01998-w
First detected geographical cluster of BoDV-1 encephalitis from same small village in two children: therapeutic considerations and epidemiological implications
Abstract
Background: The Borna disease virus (BoDV-1) is an emerging zoonotic virus causing severe and mostly fatal encephalitis in humans.
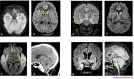
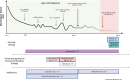
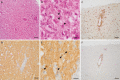
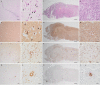
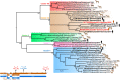
Methods and results: A local cluster of fatal BoDV-1 encephalitis cases was detected in the same village three years apart affecting two children. While the first case was diagnosed late in the course of disease, a very early diagnosis and treatment attempt facilitated by heightened awareness was achieved in the second case. Therapy started as early as day 12 of disease. Antiviral therapy encompassed favipiravir and ribavirin, and, after bioinformatic modelling, also remdesivir. As the disease is immunopathogenetically mediated, an intensified anti-inflammatory therapy was administered. Following initial impressive clinical improvement, the course was also fatal, although clearly prolonged. Viral RNA was detected by qPCR in tear fluid and saliva, constituting a possible transmission risk for health care professionals. Highest viral loads were found post mortem in the olfactory nerve and the limbic system, possibly reflecting the portal of entry for BoDV-1. Whole exome sequencing in both patients yielded no hint for underlying immunodeficiency. Full virus genomes belonging to the same cluster were obtained in both cases by next-generation sequencing. Sequences were not identical, indicating viral diversity in natural reservoirs. Specific transmission events or a common source of infection were not found by structured interviews. Patients lived 750m apart from each other and on the fringe of the settlement, a recently shown relevant risk factor.
Conclusion: Our report highlights the urgent necessity of effective treatment strategies, heightened awareness and early diagnosis. Gaps of knowledge regarding risk factors, transmission events, and tailored prevention methods become apparent. Whether this case cluster reflects endemicity or a geographical hot spot needs further investigation.
Keywords: Borna disease virus; Bornavirus; Epidemiology; Immunosuppression; Transmission; Treatment.
© 2023. The Author(s).
Conflict of interest statement
No conflict of interest concerning this publication has to be disclosed by the authors.
Figures


References
-
- Schlottau K, Forth L, Angstwurm K, Höper D, Zecher D, Liesche F, Hoffmann B, Kegel V, Seehofer D, Platen S, Salzberger B, Liebert UG, Niller HH, Schmidt B, Matiasek K, Riemenschneider MJ, Brochhausen C, Banas B, Renders L, Moog P, Wunderlich S, Seifert CL, Barreiros A, Rahmel A, Weiss J, Tappe D, Herden C, Schmidt-Chanasit J, Schwemmle M, Rubbenstroth D, Schlegel J, Pietsch C, Hoffmann D, Jantsch J, Beer M. Fatal encephalitic Borna disease virus 1 in solid-organ transplant recipients. N Engl J Med. 2018;379:1377–1379. doi: 10.1056/NEJMc1803115. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

