Association of Brain Metastases With Survival in Patients With Limited or Stable Extracranial Disease: A Systematic Review and Meta-analysis
- PMID: 36821113
- PMCID: PMC9951042
- DOI: 10.1001/jamanetworkopen.2023.0475
Association of Brain Metastases With Survival in Patients With Limited or Stable Extracranial Disease: A Systematic Review and Meta-analysis
Abstract
Importance: Intracranial metastatic disease (IMD) is a severe complication of cancer with profound prognostic implications. Patients with IMD in the setting of limited or stable extracranial disease (IMD-SE) may represent a unique and understudied subset of patients with IMD with superior prognosis.
Objective: To evaluate overall survival (OS), progression-free survival (PFS), and intracranial PFS (iPFS) in patients with IMD-SE secondary to any primary cancer.
Data sources: Records were identified from MEDLINE, EMBASE, CENTRAL, and gray literature sources from inception to June 21, 2021.
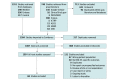
Study selection: Studies in English reporting OS, PFS, or iPFS in patients with IMD-SE (defined as IMD and ≤2 extracranial metastatic sites) and no prior second-line chemotherapy or brain-directed therapy were selected.
Data extraction and synthesis: Author, year of publication, type of study, type of primary cancer, and outcome measures were extracted. Random-effects meta-analyses were performed to estimate effect sizes, and subgroup meta-analysis and metaregression were conducted to measure between-study differences in February 2022.
Main outcomes and measures: The primary end point was OS described as hazard ratios (HRs) and medians for comparative and single-group studies, respectively. Secondary end points were PFS and iPFS.
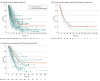
Results: Overall, 68 studies (5325 patients) were included. IMD-SE was associated with longer OS (HR, 0.52; 95% CI, 0.39-0.70) and iPFS (HR, 0.63; 95% CI, 0.52-0.76) compared with IMD in the setting of progressive extracranial disease. The weighted median OS estimate for patients with IMD-SE was 17.9 months (95% CI, 16.4-22.0 months), and for patients with IMD-PE it was 8.0 months (95% CI, 7.2-12.8 months). Pooled median OS for all patients with IMD-SE was 20.9 months (95% CI, 16.35-25.98 months); for the subgroup with breast cancer it was 20.2 months (95% CI, 10.43-38.20 months), and for non-small cell lung cancer it was 27.5 months (95% CI, 18.27-49.66 months). Between-study heterogeneity for OS and iPFS were moderate (I2 = 56.5%) and low (I2 = 0%), respectively.
Conclusions and relevance: In this systematic review and meta-analysis of patients with IMD-SE, limited systemic disease was associated with improved OS and iPFS. Future prospective trials should aim to collect granular information on the extent of extracranial disease to identify drivers of mortality and optimal treatment strategies in patients with brain metastases.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

