25-Hydroxycholesterol exacerbates vascular leak during acute lung injury
- PMID: 36821369
- PMCID: PMC10132150
- DOI: 10.1172/jci.insight.155448
25-Hydroxycholesterol exacerbates vascular leak during acute lung injury
Abstract
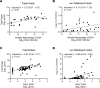
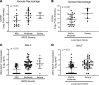
Cholesterol-25-hydroxylase (CH25H), the biosynthetic enzyme for 25-hydroxycholesterol (25HC), is most highly expressed in the lung, but its role in lung biology is poorly defined. Recently, we reported that Ch25h is induced in monocyte-derived macrophages recruited to the airspace during resolution of lung inflammation and that 25HC promotes liver X receptor-dependent (LXR-dependent) clearance of apoptotic neutrophils by these cells. Ch25h and 25HC are, however, also robustly induced by lung-resident cells during the early hours of lung inflammation, suggesting additional cellular sources and targets. Here, using Ch25h-/- mice and exogenous 25HC in lung injury models, we provide evidence that 25HC sustains proinflammatory cytokines in the airspace and augments lung injury, at least in part, by inducing LXR-independent endoplasmic reticulum stress and endothelial leak. Suggesting an autocrine effect in endothelium, inhaled LPS upregulates pulmonary endothelial Ch25h, and non-hematopoietic Ch25h deletion is sufficient to confer lung protection. In patients with acute respiratory distress syndrome, airspace 25HC and alveolar macrophage CH25H were associated with markers of microvascular leak, endothelial activation, endoplasmic reticulum stress, inflammation, and clinical severity. Taken together, our findings suggest that 25HC deriving from and acting on different cell types in the lung communicates distinct, temporal LXR-independent and -dependent signals to regulate inflammatory homeostasis.
Keywords: Cell stress; Inflammation; Innate immunity; Mouse models; Pulmonology.
Figures
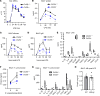
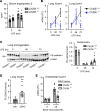
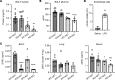
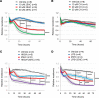

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL076259/HL/NHLBI NIH HHS/United States
- K23 HL144916/HL/NHLBI NIH HHS/United States
- P50 HL073996/HL/NHLBI NIH HHS/United States
- R01 GM122940/GM/NIGMS NIH HHS/United States
- R01 HL146829/HL/NHLBI NIH HHS/United States
- R01 HL087823/HL/NHLBI NIH HHS/United States
- R01 HL152761/HL/NHLBI NIH HHS/United States
- R01 HL155051/HL/NHLBI NIH HHS/United States
- P30 DK127984/DK/NIDDK NIH HHS/United States
- UL1 TR003163/TR/NCATS NIH HHS/United States
- P01 HL160487/HL/NHLBI NIH HHS/United States
- Z01 ES102005/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

