CD11c+ macrophages are proangiogenic and necessary for experimental choroidal neovascularization
- PMID: 36821388
- PMCID: PMC10132149
- DOI: 10.1172/jci.insight.168142
CD11c+ macrophages are proangiogenic and necessary for experimental choroidal neovascularization
Abstract
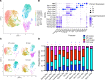
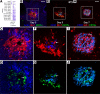
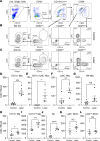
Patients with neovascular AMD (nAMD) suffer vision loss from destructive angiogenesis, termed choroidal neovascularization (CNV). Macrophages are found in CNV lesions from patients with nAMD. Additionally, Ccr2-/- mice, which lack classical monocyte-derived macrophages, show reduced CNV size. However, macrophages are highly diverse cells that can perform multiple functions. We performed single-cell RNA-Seq on immune cells from WT and Ccr2-/- eyes to uncover macrophage heterogeneity during the laser-induced CNV mouse model of nAMD. We identified 12 macrophage clusters, including Spp1+ macrophages. Spp1+ macrophages were enriched from WT lasered eyes and expressed a proangiogenic transcriptome via multiple pathways, including vascular endothelial growth factor signaling, endothelial cell sprouting, cytokine signaling, and fibrosis. Additionally, Spp1+ macrophages expressed the marker CD11c, and CD11c+ macrophages were increased by laser and present in CNV lesions. Finally, CD11c+ macrophage depletion reduced CNV size by 40%. These findings broaden our understanding of ocular macrophage heterogeneity and implicate CD11c+ macrophages as potential therapeutic targets for treatment-resistant patients with nAMD.
Keywords: Inflammation; Macrophages; Ophthalmology; Retinopathy.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

