The development of a rapid patient-derived xenograft model to predict chemotherapeutic drug sensitivity/resistance in malignant glial tumors
- PMID: 36821432
- PMCID: PMC10479744
- DOI: 10.1093/neuonc/noad047
The development of a rapid patient-derived xenograft model to predict chemotherapeutic drug sensitivity/resistance in malignant glial tumors
Abstract
Background: High-grade gliomas (HGG) are aggressive brain tumors associated with short median patient survival and limited response to therapies, driving the need to develop tools to improve patient outcomes. Patient-derived xenograft (PDX) models, such as mouse PDX, have emerged as potential Avatar platforms for personalized oncology approaches, but the difficulty for some human grafts to grow successfully and the long time required for mice to develop tumors preclude their use for HGG.
Methods: We used a rapid and efficient ex-ovo chicken embryo chorioallantoic membrane (CAM) culture system to evaluate the efficacy of oncologic drug options for HGG patients.
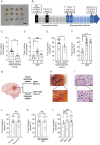
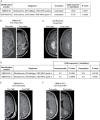
Results: Implantation of fresh glioma tissue fragments from 59 of 60 patients, that include difficult-to-grow IDH-mutated samples, successfully established CAM tumor xenografts within 7 days, with a tumor take rate of 98.3%. These xenografts faithfully recapitulate the histological and molecular characteristics of the primary tumor, and the ability of individual fragments to form tumors was predictive of poor patient prognosis. Treatment of drug-sensitive or drug-resistant xenografts indicates that the CAM-glioma assay enables testing tumor sensitivity to temozolomide and carboplatin at doses consistent with those administered to patients. In a proof-of-concept study involving 14 HGG patients, we observed a correlation of 100% between the CAM xenograft response to temozolomide or carboplatin and the clinical response of patients.
Conclusion: The CAM-glioma model is a fast and reliable assay that has the potential to serve as a complementary model to drug discovery and a real-time Avatar platform to predict the best treatment for HGG patients.
Keywords: Avatar model; chick chorioallantoic membrane assay; glioblastoma; patient-derived xenograft model; personalized medicine.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures



References
-
- Stupp R, Brada M, van den Bent MJ, Tonn J-C, Pentheroudakis G, ESMO Guidelines working group. high-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iiiiii9393–iii101. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Stupp R, Taillibert S, Kanner AA, et al. . Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

