TGF-β in the microenvironment induces a physiologically occurring immune-suppressive senescent state
- PMID: 36821441
- PMCID: PMC10187541
- DOI: 10.1016/j.celrep.2023.112129
TGF-β in the microenvironment induces a physiologically occurring immune-suppressive senescent state
Abstract
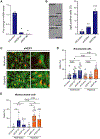
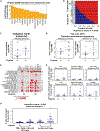
TGF-β induces senescence in embryonic tissues. Whether TGF-β in the hypoxic tumor microenvironment (TME) induces senescence in cancer and how the ensuing senescence-associated secretory phenotype (SASP) remodels the cellular TME to influence immune checkpoint inhibitor (ICI) responses are unknown. We show that TGF-β induces a deeper senescent state under hypoxia than under normoxia; deep senescence correlates with the degree of E2F suppression and is marked by multinucleation, reduced reentry into proliferation, and a distinct 14-gene SASP. Suppressing TGF-β signaling in tumors in an immunocompetent mouse lung cancer model abrogates endogenous senescent cells and suppresses the 14-gene SASP and immune infiltration. Untreated human lung cancers with a high 14-gene SASP display immunosuppressive immune infiltration. In a lung cancer clinical trial of ICIs, elevated 14-gene SASP is associated with increased senescence, TGF-β and hypoxia signaling, and poor progression-free survival. Thus, TME-induced senescence may represent a naturally occurring state in cancer, contributing to an immune-suppressive phenotype associated with immune therapy resistance.
Keywords: CP: Cancer; CP: Immunology; E2Fs; SASP; TGF-β; hypoxia; immune checkpoint inhibitors; lung cancer; senescence; tumor microenvironment.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A. Ravi has served as a consultant to Halo Solutions and Tyra Biosciences. J.F.G. has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Moderna, Mirati, AstraZeneca, Pfizer, Novartis, iTeos, Nuvalent, Karyopharm, Beigene, Silverback Therapeutics, Merck, and GlydeBio; received research support from Novartis, Genentech/Roche, and Ariad/Takeda; received institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. D.T.T. has received consulting fees from ROME Therapeutics, Tekla Capital, Ikena Oncology, Foundation Medicine, Inc., NanoString Technologies, and Pfizer that are not related to this work. D.T.T. is a founder of and has equity in ROME Therapeutics, PanTher Therapeutics, and TellBio, Inc., which are not related to this work. D.T.T. receives research support from ACD-Biotechne, PureTech Health LLC, and Ribon Therapeutics, which was not used in this work. D.T.T.’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. S. Maheswaran and D.A.H. are co-founders and have equity in Tell-Bio, which is not related to this work.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

