Rapid Single-Shot Synthesis of the 214 Amino Acid-Long N-Terminal Domain of Pyocin S2
- PMID: 36821521
- PMCID: PMC10460144
- DOI: 10.1021/acschembio.2c00862
Rapid Single-Shot Synthesis of the 214 Amino Acid-Long N-Terminal Domain of Pyocin S2
Abstract
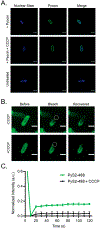
The impermeable outer membrane of Pseudomonas aeruginosa is bypassed by antibacterial proteins known as S-type pyocins. Because of their properties, pyocins are investigated as a potential new class of antimicrobials against Pseudomonas infections. Their production and modification, however, remain challenging. To address this limitation, we employed automated fast-flow peptide synthesis for the rapid production of a pyocin S2 import domain. The N-terminal domain sequence (PyS2NTD) was synthesized in under 10 h and purified to yield milligram quantities of the desired product. To our knowledge, the 214 amino acid sequence of PyS2NTD is among the longest peptides produced from a "single-shot" synthesis, i.e., made in a single stepwise route without the use of ligation techniques. Biophysical characterization of the PyS2NTD with circular dichroism was consistent with the literature reports. Fluorescently labeled PyS2NTD binds to P. aeruginosa expressing the cognate ferripyoverdine receptor and is taken up into the periplasm. This selective uptake was validated with confocal and super resolution microscopy, flow cytometry, and fluorescence recovery after photobleaching. These modified, synthetic S-type pyocin domains can be used to probe import mechanisms of P. aeruginosa and leveraged to develop selective antimicrobial agents that bypass the outer membrane.
Figures


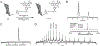
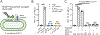
References
-
- Tan Y; Wu H; Wei T; Li X Chemical Protein Synthesis: Advances, Challenges, and Outlooks. J. Am. Chem. Soc 2020, 142 (48), 20288–20298. - PubMed
-
- Kulkarni SS; Sayers J; Premdjee B; Payne RJ Rapid and Efficient Protein Synthesis through Expansion of the Native Chemical Ligation Concept. Nat. Rev. Chem 2018, 2 (4), 0122.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

