Cardiac Manifestations in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD): A Single-Center Study
- PMID: 36821607
- PMCID: PMC10103268
- DOI: 10.34067/KID.0002942022
Cardiac Manifestations in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD): A Single-Center Study
Abstract
Key Points:
Cardiovascular disease—a key driver of morbidity in CKD—is common in patients with autosomal dominant polycystic kidney disease (ADPKD).
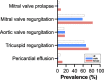
Pathologic echocardiography findings, including valvular defects, aortic root dilation, and hypertrophy, are found in most patients with ADPKD.
These findings correlate with parameters indicating disease progression in ADPKD. Echocardiography should be offered to all patients with ADPKD.
Background: ADPKD is the most common monogenetic kidney disease and results in kidney failure in >75% of affected individuals. As a systemic disorder, ADPKD is associated with a variety of extrarenal manifestations, including cardiac manifestations, that affect the majority of patients. We characterized the cardiac involvement in patients with ADPKD from the German AD(H)PKD registry and compared them with kidney donor candidates as controls.
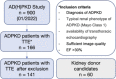
Methods: In this single-center cohort study, we evaluated 141 patients with ADPKD (44.17±11.23 years) from the German AD(H)PKD registry and 60 kidney donor candidates (55.08±10.21 years). All patients underwent clinical examination, abdominal MRI, and transthoracic echocardiography.
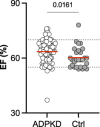
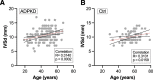
Results: Of the patients with ADPKD, 65% showed hypertrophy of the left ventricle (as defined by an end-diastolic interventricular septal wall thickness [IVSd] >10 mm) compared with 55% in control patients. Mitral regurgitation was the most common finding among 54% of patients with ADPKD who exhibited valvular dysfunction, albeit mild in most patients. Interestingly, left ventricular ejection fraction (LV-EF) differed significantly between both groups, with higher values in patients with ADPKD (64%±6% versus 60%±6%), whereas other parameters, including IVSd, left ventricular end-diastolic diameter (LVEDD), tricuspid annular plane systolic excursion (TAPSE), and pressure gradients across the aortic and tricuspid valve were similar between groups. Correlations of echocardiographic parameters with markers of disease progression revealed statistically significant associations for aortic root diameter (P=0.01), the pressure gradient across the aortic valve (AV dPmax; P=0.0003), and IVSd (P=0.0001), indicating rapid kidney disease progression may also be associated with cardiac findings.
Conclusion: Cardiovascular abnormalities are prevalent in patients with ADPKD. Considering the importance of cardiovascular disease for outcomes in CKD, early management and possibly prevention are important goals of any treatment scheme. Consequently, echocardiography should be offered to all patients with ADPKD in routine management.
Conflict of interest statement
R.-U. Müller reports having consultancy agreements with, and receiving honoraria from, Alnylam and Sanofi; having ownership interest in Bayer, ChemoCentryx, Novartis, Pfizer, Roche, and Santa Barbara Nutrients; serving as chair of the working group "Genes and Kidney" of the European Renal Association (ERA) and member of the working group on AD dysplasias of the European Rare Kidney Disease Reference Network (ERKNet), on the editorial board of
Figures




Comment in
-
Defining Cardiac Dysfunction in ADPKD.Kidney360. 2023 Feb 1;4(2):126-127. doi: 10.34067/KID.0000000000000066. Kidney360. 2023. PMID: 36821601 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

