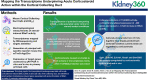
Mapping the Transcriptome Underpinning Acute Corticosteroid Action within the Cortical Collecting Duct
- PMID: 36821614
- PMCID: PMC10103384
- DOI: 10.34067/KID.0003582022
Mapping the Transcriptome Underpinning Acute Corticosteroid Action within the Cortical Collecting Duct
Abstract
Key Points:
We report the transcriptomes associated with acute corticosteroid regulation of ENaC activity in polarized mCCDcl1 collecting duct cells.
Nine genes were regulated by aldosterone (ALDO), 0 with corticosterone alone, and 151 with corticosterone when 11βHSD2 activity was inhibited.
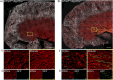
We validated three novel ALDO-induced genes, Rasd1, Sult1d1, and Gm43305, in primary cells isolated from a novel principal cell reporter mouse.
Background: Corticosteroids regulate distal nephron and collecting duct (CD) Na+ reabsorption, contributing to fluid-volume and blood pressure homeostasis. The transcriptional landscape underpinning the acute stimulation of the epithelial sodium channel (ENaC) by physiological concentrations of corticosteroids remains unclear.
Methods: Transcriptomic profiles underlying corticosteroid-stimulated ENaC activity in polarized mCCDcl1 cells were generated by coupling electrophysiological measurements of amiloride-sensitive currents with RNAseq. Generation of a principal cell-specific reporter mouse line, mT/mG-Aqp2Cre, enabled isolation of primary CD principal cells by FACS, and ENaC activity was measured in cultured primary cells after acute application of corticosteroids. Expression of target genes was assessed by qRT-PCR in cultured cells or freshly isolated cells after the acute elevation of steroid hormones in mT/mG-Aqp2Cre mice.
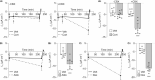
Results: Physiological relevance of the mCCDcl1 model was confirmed with aldosterone (ALDO)-specific stimulation of SGK1 and ENaC activity. Corticosterone (CORT) only modulated these responses at supraphysiological concentrations or when 11βHSD2 was inhibited. When 11βHSD2 protection was intact, CORT caused no significant change in transcripts. We identified a small number of ALDO-induced transcripts associated with stimulated ENaC activity in mCCDcl1 cells and a much larger number with CORT in the absence of 11βHSD2 activity. Principal cells isolated from mT/mG-Aqp2Cre mice were validated and assessment of identified ALDO-induced genes revealed that Sgk1, Zbtbt16, Sult1d1, Rasd1, and Gm43305 are acutely upregulated by corticosteroids both in vitro and in vivo.
Conclusions: This study reports the transcriptome of mCCDcl1 cells and identifies a small number of ALDO-induced genes associated with acute stimulation of ENaC, including three previously undescribed genes.
Conflict of interest statement
M.A. Bailey reports the following: Consultancy: River 2 Renal; and Advisory or Leadership Role: Kidney Research UK (Research Grant & Fellowships Committee);
Figures






Comment in
-
How Does Aldosterone Work?Kidney360. 2023 Feb 1;4(2):131-133. doi: 10.34067/KID.0000000000000058. Kidney360. 2023. PMID: 36821603 Free PMC article. No abstract available.

