Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes
- PMID: 36821708
- PMCID: PMC9945580
- DOI: 10.1289/EHP11072
Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes
Abstract
Background: Microplastics (MPs) are small particles of plastic ( in diameter). In recent years, oral exposure to MPs in living organisms has been a cause of concern. Leaky gut syndrome (LGS), associated with a high-fat diet (HFD) in mice, can increase the entry of foreign substances into the body through the intestinal mucosa.
Objectives: We aimed to evaluate the pathophysiology of intestinal outcomes associated with consuming a high-fat diet and simultaneous intake of MPs, focusing on endocrine and metabolic systems.
Methods: C57BL6/J mice were fed a normal diet (ND) or HFD with or without polystyrene MP for 4 wk to investigate differences in glucose tolerance, intestinal permeability, gut microbiota, as well as metabolites in serum, feces, and liver.
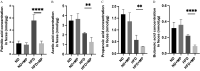
Results: In comparison with HFD mice, mice fed the HFD with MPs had higher blood glucose, serum lipid concentrations, and nonalcoholic fatty liver disease (NAFLD) activity scores. Permeability and goblet cell count of the small intestine (SI) in HFD-fed mice were higher and lower, respectively, than in ND-fed mice. There was no obvious difference in the number of inflammatory cells in the SI lamina propria between mice fed the ND and mice fed the ND with MP, but there were more inflammatory cells and fewer anti-inflammatory cells in mice fed the HFD with MPs in comparison with mice fed the HFD without MPs. The expression of genes related to inflammation, long-chain fatty acid transporter, and cotransporter was significantly higher in mice fed the HFD with MPs than in mice fed the HFD without MPs. Furthermore, the genus Desulfovibrio was significantly more abundant in the intestines of mice fed the HFD with MPs in comparison with mice fed the HFD without MPs. Muc2 gene expression was decreased when palmitic acid and microplastics were added to the murine intestinal epithelial cell line MODE-K cells, and Muc2 gene expression was increased when IL-22 was added.
Discussion: Our findings suggest that in this study, MP induced metabolic disturbances, such as diabetes and NAFLD, only in mice fed a high-fat diet. These findings suggest that LGS might have been triggered by HFD, causing MPs to be deposited in the intestinal mucosa, resulting in inflammation of the intestinal mucosal intrinsic layer and thereby altering nutrient absorption. These results highlight the need for reducing oral exposure to MPs through remedial environmental measures to improve metabolic disturbance under high-fat diet conditions. https://doi.org/10.1289/EHP11072.
Figures







References
-
- Plastics-the Facts 2018 An analysis of European plastics production, demand and waste data.
-
- UNEP (United Nations Environmental Programme). Single-Use Plastics: A Roadmap for Sustainability | International Environmental Technology Centre. https://www.unep.org/ietc/ja/node/53?%2Fresources%2Fpublication%2Fsingle... [accessed 3 May 2022].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

