Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma
- PMID: 36821768
- PMCID: PMC10646788
- DOI: 10.1182/blood.2022018893
Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma
Abstract
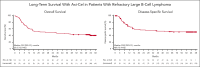
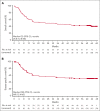
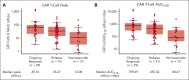
In phase 2 of ZUMA-1, a single-arm, multicenter, registrational trial, axicabtagene ciloleucel (axi-cel) autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy demonstrated durable responses at 2 years in patients with refractory large B-cell lymphoma (LBCL). Here, we assessed outcomes in ZUMA-1 after 5 years of follow-up. Eligible adults received lymphodepleting chemotherapy followed by axi-cel (2 × 106 cells per kg). Investigator-assessed response, survival, safety, and pharmacokinetics were assessed in patients who had received treatment. The objective response rate in these 101 patients was 83% (58% complete response rate); with a median follow-up of 63.1 months, responses were ongoing in 31% of patients at data cutoff. Median overall survival (OS) was 25.8 months, and the estimated 5-year OS rate was 42.6%. Disease-specific survival (excluding deaths unrelated to disease progression) estimated at 5 years was 51.0%. No new serious adverse events or deaths related to axi-cel were observed after additional follow-up. Peripheral blood B cells were detectable in all evaluable patients at 3 years with polyclonal B-cell recovery in 91% of patients. Ongoing responses at 60 months were associated with early CAR T-cell expansion. In conclusion, this 5-year follow-up analysis of ZUMA-1 demonstrates sustained overall and disease-specific survival, with no new safety signals in patients with refractory LBCL. Protracted B-cell aplasia was not required for durable responses. These findings support the curative potential of axi-cel in a subset of patients with aggressive B-cell lymphomas. This trial was registered at ClinicalTrials.gov, as #NCT02348216.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.S.N. received consulting fees or honoraria from Kite, Merck, Bristol-Myers Squibb (BMS), Novartis, Allogene Therapeutics, Cell Medica/Kuur/Athenex, Incyte, Legend Biotech, Adicet Bio, bluebird bio, Sellas Life Sciences, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Medscape, Aptitude Health, Bio Ascend, and MJH Life Sciences; grants, contracts, or research funding from Kite, BMS, Poseida, Cellectis, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, and Adicet Bio; stock options from Longbow Immunotherapy; and patents, royalties, or other intellectual property from Takeda Pharmaceuticals, related to cell therapy. C.A.J. received honoraria from Kite, Novartis, BMS/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi-Sankyo; performed a consulting/advisory role for Kite, Novartis, BMS/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi-Sankyo; and received research funding from Kite and Pfizer. A.G. performed a consulting/advisory role for Amgen, Atara, Celgene, Kite, and Wugen Inc; received research funding from Amgen and Kite; and honoraria from Kite. D.B.M. received honoraria and research funding from Janssen; performed a consulting/advisory role for Janssen, Adaptive Biotechnologies, Kite, BMS, and Miltenyi; received patents, royalties, other intellectual property as a cGVHD patent holder for ibrutinib as cGVHD therapy with no compensation; and travel and other support from Janssen. O.O.O. performed a consultancy role and participated in an advisory board for Pfizer, Kite, Gilead Sciences, AbbVie, Janssen, TGR Therapeutics, ADC, Novartis, Epizyme, Curio Science, Nektar, and Syncopation; and received honoraria from Pfizer and Gilead Sciences. Y.L. performed a consulting/advisory role for Kite, BMS, bluebird bio, Janssen, Legend BioTech, Gamida Cells, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; and received research funding from Kite, BMS, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. B.H. received honoraria from, performed a consulting/advisory role for, and received research funding and travel support from Kite. J.M.T. performed a consulting/advisory role for Kite; and received research funding from BMS, Kite, Merck, and Spectrum. A.D. performed a consulting/advisory role for Adicet, Janssen, and Kite. P.M.R. performed a consulting/advisory role for Kite; and received research funding from Genentech and Seagen. P.S. received honoraria from and performed a consulting/advisory role for MorphoSys and CRISPR Therapeutics; and received research funding from Amgen, Gamida Cell, Pfizer, Karyopharm, Gilead Sciences, Incyte, Seagen, and Cellectar. I.W.F. performed a consulting/advisory role for AbbVie, AstraZeneca, BeiGene, Century Therapeutics, Genentech, Genmab, Gilead Sciences, Great Point Partners, Hutchison MediPharma, Iksuda Therapeutics, InnoCare Pharma, Janssen, Juno Therapeutics, Kite, MorphoSys, Novartis, Nurix Therapeutics, Pharmacyclics, Roche, Seagen, Servier Pharmaceuticals, Takeda, TG Therapeutics, Unum Therapeutics, Verastem, Vincerx Pharma, and Yingli Pharmaceuticals; and received research funding from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Curis, Forma Therapeutics, Forty Seven, Genentech, Gilead Sciences, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Juno Therapeutics, Karyopharm Therapeutics, Kite, Loxo, Merck, MorphoSys, Novartis, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Roche, Seagen, Takeda, Teva, TG Therapeutics, Trillium Therapeutics, Triphase Research and Development Corp, Unum Therapeutics, and Verastem. U.F. received honoraria from Kite; performed a consulting/advisory role for MorphoSys; and received research funding from Checkmate Pharma. A.G. was employed with Regional Cancer Care Associates and OMI; had a leadership role with COTA Healthcare and Genomics Testing Cooperative LLC; had stock or other ownership in COTA Healthcare, Genomics Testing Cooperative LLC, Alloplex, and Resilience; performed a consulting/advisory role for Kite, Pharmacyclics, Janssen, Clinical Advances in Hematology & Oncology, Michael J. Hennessey Associates, Inc, Physicians Education Resource; received research funding from Acerta, AstraZeneca, BMS, Celgene, Constellation, Genentech, Genentech-Hoffman, Roche, Infinity, Infinity Pharmaceuticals, Verastem, Janssen, Karyopharm, Kite, MorphoSys, Pharmacyclics, Seagen, and Verastem; travel support from Physcians Education Resource; participated in scientific advisory boards with BMS, Alloplex, and Vincerx; and participated in steering committees with AstraZeneca, Pharmacyclics, and Janssen. P.A.M. received honoraria from, performed a consulting/advisory role for, and participated in a speakers' bureau for Kite; and research funding from AlloVir, AutolusTherapeutics, Kite, and Novartis. J.M. had a consulting/advisory role for Pharmacyclics/AbbVie, Bayer, Kite, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seagen, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, BeiGene, Servier, Novartis, MorphoSys/Incyte, Mei Pharma, and Zodiac; received research funding from Bayer, Kite, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seagen, Janssen, and Millennium; honoraria from Targeted Oncology, OncView, Curio, Kyowa, Physicians’ Education Resource, Dava, Global Clinical Insights, MJH, Shanghai Youyao, and Seagen; and participated in a speakers’ bureau with Kite, Kyowa, Bayer, Pharmacyclics/Janssen, Seagen, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Celgene/BMS, and Genentech/Roche. T.S. performed a consultancy or advisory role for AbbVie, AstraZeneca, BeiGene, Celgene, Juno, Kite, and PCYC; participated in a speakers’ bureau with AstraZeneca, BeiGene, and BMS; and received institutional research funding from Ascentage Pharma, AstraZeneca, BeiGene, BMS, Celgene, Juno, Kite, Oncternal, PCYC, and TG Therapeutics. J.C.C. received honoria from BeiGene, AstraZeneca, and Epyzime; performed a consulting/advisory role for AbbVie, Kite, Novartis, BMS, TG Therapeutics, and TeneBio; and received research funding from Adaptive, Merck, Janssen, ADC Therapeutics, and AstraZeneca. A.F.H. performed a consulting/advisory role for ADC Therapeutics, AstraZeneca, BMS, Genentech, Karyopharm, Merck, Seagen, Takeda, and Tubulis; and received research funding from ADC Therapeutics, AstraZeneca, BMS, Genentech, Gilead Sciences, Karyopharm, Kite, Merck, and Seagen. N.L.B. performed a consulting/advisory role for ADC Therapeutics, Roche/Genentech, and Seagen; and received research funding from ADC Therapeutics, Autolus, BMS, Celgene, Forty Seven, Genentech, Janssen, Kite, Merck, Millennium, Pharmacyclics, and Seagen. A.A.B. was a former employee with Kite; currently employed with Capstan Therapeutics; with stock or other ownership with Capstan Therapeutics and Gilead Sciences; performed a consulting/advisory role for Cero Therapeutics and Elicio; and provided expert testimony for Gilead Sciences. R.R.S. is employed with, and has stock or other ownership at Kite; and received patents, royalties, and other intellectual property from Atara and Kite. J.D. is employed at Kite; with stock or other ownership in Gilead Sciences; performed a consulting/advisory role for GliaCure; and received patents, royalties, or other intellectual property from Patent US8598141 (3 December 2013). K.S., J.J.K., and Y.Z. have been employed with and stock or other ownership at Kite. H.M. has been employed at Kite and Takeda; with stock or ownership in Kite and Gilead Sciences. F.L.L. performed a consulting/advisory role for Allogene, Amgen, bluebird bio, BMS, Calibr, Cellular Biomedicine Group, Cowen, ecoR1, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, Legend Biotech, Novartis, Umoja, and Wugen; received research funding from Allogene, Kite, and Novartis; and the institution holds patents, royalties, other intellectual property from several patents in author’s name (unlicensed) in the field of cellular immunotherapy. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Putting the pedal to the metal: axi-cel for LBCL.Blood. 2023 May 11;141(19):2285-2286. doi: 10.1182/blood.2023020188. Blood. 2023. PMID: 37166931 No abstract available.
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- YESCARTA® (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2022.
-
- YESCARTA® (axicabtagene ciloleucel). Summary of product characteristics. Kite Pharma EU B.V.; 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

